Topics
Session 3: Diastology and Cardiomyopathies
Session 4: Pulmonary Hypertension and Diseases of the Aorta
Session 5: Pericardial Disease
Session 6: Ischaemic Heart Disease and Stress Echo
Masterclass: Sonographer Basics
Masterclass: Sonographer Advanced
Masterclass: Adult Congenital Heart Disease Basics
Session 1: Guideline Update
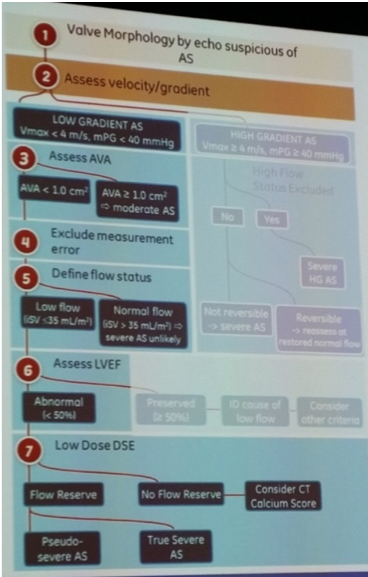
B Anderson – echocardiographic assessment of aortic stenosis
- AS severity, any 1 of 3 can suggest AS:
- Vmax
- Mean PG
- AVA via continuity equation = (CSA LVOT * VTI LVOT)/VTI AV
- Dimensionless severity index (DSI) should be consistent with AS severity
- Discordant data may result from: technical errors (as below) and body size (overcome by indexing by BSA)
- LVOT diameter is the greatest potential error in continuity equation for calculation of AVA, tips for measurement:
- PLAX no longer require RCC and NCC visualisation (results in transection), correct view RCC and hinge point between LCC and NCC
- Alternative predicted LVOTd = (5.7 * BSA) + 12.1
- AS jets are eccentric, so must interrogate from multiple windows (Thaden JJ et al 2015 Jul;28(7):780-5): highest AS jet velocity from RSE
- LVOT diameter is the greatest potential error in continuity equation for calculation of AVA, tips for measurement:
- New classification of AS guided by:
- Gradients – peak AS velocity >4m/s predictor of worse prognosis
- LVEF, and
- Flow: indexed SV (mL/m2) = (CSA LVOT * VTI LVOT)/BSA (m2)
- High flow >58ml/m2
- Low flow <35ml/m2 – either a) classical low flow (depressed EF<50% valve or ventricle), or b) paradoxical low flow (LVEF>50%, numerous causes resulting in reduced transvalvular gradient e.g. MR)
- In conjunction with BP, symptoms, physical examination, AV appearance and AVA:
M Bremer – non-invasive evaluation of native valvular regurgitation (technical)
- ASE guidelines and standards (J Am Soc Echocardophaphy April 2017)
- Mechanisms of MR:
- Organic (primary): structural alteration of valvular apparatus
- Degenerative – calcification, thickening
- Infective – endocarditis, perforations, aneurysm
- Inflammatory – rheumatic, collagen vascular disease, radiation
- Myxomatous – prolapse, flail, ruptured or elongated chordae, ruptured papillary muscle (typically acute MR)
- Congenital – cleft leaflet, parachute MV
- Functional (secondary): structurally normal valve with cardiac chamber remodelling (incomplete coaptation of leaflets)
- Non-ischaemic dilated CM
- Ischaemic mitral regurgitation
- Annular dilatation
- Carpentier (functional) classification
- Type I (normal): annular dilatation, leaflet perforation
- Type II (increased): prolapse, chordal elongation or rupture, PM rupture
- Type III (restricted): rheumatic, ischaemic, functional
- Mechanical impact of regurgitation: Mitral
- Chronic MR: volume overload and LV+LA enlargement, systolic atrial septum bowing to Rt, Rt heart chambers and pulmonary HTN, depressed LV function (timing surgical intervention dependent on regurgitant volume and LV size and function)
- Severe acute MR: hyperdynamic LV function
- Mechanisms of AR:
- Organic (primary): leaflet abnormalities
- Biscuspid aortic valve
- VSD (disruption of aortic root changes coaptation)
- Rheumatic disease
- Endocarditis
- Functional (secondary): aortic root abnormalities
- Aortic aneurysm / dilated annulus
- Aortic dissection
- Mechanical impact of regurgitation: Aortic
- Chronic AR: LV volume hypertrophy (increase LV mass and size), with subsequent reduction in LVEF
- Severity of regurgitation (qualitative)
- Colour doppler imaging useful assess origin, spatial orientation in receiving chamber, and flow convergence (caution pitfalls) – see guidelines for values e.g. MR:
- Mild: small, central, often brief
- Moderate: between mild-large
- Large: >50% of LA area or eccentric jet
- Doppler e.g. MR:
- Predominantly early filling, Evelocity >1.2-15m/second consistent with severe MR in absence of LV diastolic dysfunction,
- Pulmonary veins: normal, bunted or systolic reversal
- CWD: density proportional to regurgitant volume
- Colour doppler imaging useful assess origin, spatial orientation in receiving chamber, and flow convergence (caution pitfalls) – see guidelines for values e.g. MR:
- Severity of regurgitation (quantitative): see ‘Masterclass Sonographer Advanced’
- Volumetric method,
- PISA (flow convergence)
- Organic (primary): leaflet abnormalities
- Organic (primary): structural alteration of valvular apparatus
D Burstow – appropriate use of criteria for valvular heart disease (application)
- General principles
- Comprehensive imaging – 2D and 3D (superior spatial imaging of lesions) imaging to assess aetiology (primary or functional), and mechanism (Carpentier classification), which is important to determine complications and management
- Integrative interpretation – intrinsic limitations to all methods (algorithm per: J Am Soc Echocardiography. 2017 Apr;30(4):303-371) for both qualitative and quantitative methods, particularly eccentric jets
- Individualisation – for example, pressure half-time to assess ventricular physiology
- Appropriate use and application – additional testing available e.g. TOE and CMR (TTE baseline, TOE additional MV analysis, CMR quantification in AR and RV size and function)
J Chan – incorporating strain imaging into routine clinical practice
- Strain is deformation resulting from applied force:
= [(Length at point time – Length baseline)/Length baseline] x100 - Measures microscopic deformation: quantitative AND vector (magnitude and direction)
- Reliance on unique acoustic speckle (interaction of US beam with tissue) movement over time
- Strain can be positive (if lengthening) or negative (if shortening) depending on direction:
- Longitudinal: Systole negative (shortening), diastole positive (lengthening)
- Radial: Systole positive (thickening), diastole negative (thinning)
- Circumferential: Systole negative (shortening), diastole positive (lengthening)
- Most common application: LV global longitudinal strain (GLS), via apical 3C, 2C and 4C
- Display: bull’s eye plot (17 segment model), strain curve, segmental parametric view, or curved anatomical colour M-mode (for temporal relationship)
- Incorporation and standardisation (European Heart Journal guideline, cardiovascular imaging 2015) to improve utilisation of strain imaging:
- Complexity standardisation:
- GLS >20% normal <20% abnormal
- Timing (of systolic strain): end systolic strain (ESS)=point of AV closure
- Calculate global strain (as opposed to segmental)
- Standardisation (Yingchoncharoen et al JASE 2013):
- Normal strain value (longitudinal, circumferential, radial) – radial strain mean values vary significantly (currently not useful), strain values dependent on load (preload/afterload dependent), age (older>lower strain), gender (female>slightly higher strain), inter-vendors variability
- New GLS suggestions (not in guidelines): Very severe <8% / severe <12% / reduced <12-16% / borderline 16-18% / normal 18-20% / supranormal GLS >20% – note that can’t compare to EF as assessing different values
- Serial assessment: utilise same vendor’s equipment and same version software because of intervendor compatibility
- Precise/reliable results:
- Image and tracking quality – 2D speckles tracking, good image quality (should not be performed if can’t visualise 2 or more myocardial segments), need high frame rates (40-80 – too low miss temporal resolution, too high miss spatial resolution)
- GLS: 50 cases competency in GLS analysis, greater learning curve for GCS and GRS
- Applications:
- Subclinical dysfunction – correlates well with LVEF, optimal parameter to detect early subclinical dysfunction e.g. chemotherapy for breastCa e.g.2. RV strain to predict those requiring a BiVAD (as opposed to LVAD alone)
- Pattern recognition (regional strain) – LVH aetiology
- Future:
- Left atrial strain as a potential marker of LV diastolic dysfunction; 2C and 4C view, reservoir function (MVC-MVO), conduit function (MVO-LA contraction), contractile function (LA contraction-MVC)
- RV longitudinal strain: focused 4C view free wall only, RVFWS 20% cut-off
- Myocardial work: LV pressure-strain relations (Chan et al, Eur Heart J 2018)
- Complexity standardisation:
J Lindner – clinical applications of ultrasonic enhancing agents (UEA)
- US contrast agents: encapsulated microbubbles with albumin or lipid shell (2-5microns diameter), high MW gas (non-diffusing), signal produced by volumetric oscillation
- US stimulation of microbubbles > volumetric oscillation (cavitation) according to acoustic pressure > detection of non-linear oscillation (broadband signal)
- Approved usage: LV opacification assess LV size and function
- Off label: utilise UEAs when most impactful to improve diagnostic capability, particularly if poorer views, particularly when examining for apical diseases (thrombus, hypertrophic apical CM, eosinophilic apical CM), RWMAs (?angina ?inducible ischaemia on stress echo)
Session 2: Valves
H Thomson – when 3D is most useful
- Useful for: LV size and EF, and valvular disease
- For example, anatomic analysis of valve (MVR) pathology:
- Lesion localisation including segmental analysis, specific mechanism (Carpentier’s classification limited) and severity (vena contracta, 3D PISA)
- Important to guide interventions peri-operatively, for example:
- Surgical repair: P2 in DMR
- Mitraclip: A2 and P2 in DMR and functional MR
A Luis – assessing mitral stenosis: pearls and pitfalls
- EAE/ASE guidelines (diagnosis) and AHA/ACC guidelines (management)
- MS classification: severity important to guide monitoring and management
- Previous guidelines: Nisiumura RA et al Circulation 2008, based on mean gradient (moderate: 5-10), PASP (moderate: 30-50), and valve area (moderate: 1.0-1.5)
- Elevated MG does not always imply MS: can also occur in severe MR, high output states or high heart rate
- New classification inclusive of symptomatology, mean gradient removed as defining feature:
- At risk of MS: mild valve doming
- Progressive or less than severe MS: MV area >1.5cm2
- Severe MS: MV area <1.5cm2, PHT >150ms
- Very severe MS: MV area <1.0cm2, PHT>220ms
- MVA calculation
- Planimetry: zoomed PSAX image, measure from leaflet tips inclusive commissures, at mid-diastole, biplane or 3D helpful
- Pressure half-time: time interval to reach half peak pressure, utilise CWD, where MVA = 220/PHT
- Unreliable if altered compliance of LA or LV e.g. immediately post PMBV, severe AR, decreased LV compliance, degenerative MS
- Continuity equation: where MVA = SV/MV TVI, relies on principle of constant flow so unable use if AF or significant MR or AR
- PISA (proximal isovelocity surface area): ERO = [(6.28 x r2 x Valiasing)/Vmax] x (angle/180)
- Other echocardiographic assessment:
- Stress echocardiography: assess MR and TR
- Severe MS if MG>15mmHg with exercise
- >18mmHg with dobutamine
- Percutaneous balloon valvuloplasty: Wilkins score used to assess suitability for valvuloplasty, based on mobility, thickening, calcification, subvalvular thickening, score each 1-4 each, total score <9 consider valvuloplasty, if >8 unlikely to help, note also consider severity of commissural calcification (if severe likely PBM won’t help)
- Rheumatic MS (tips to annulus): favourable to balloon valvuloplasty if no LA clot and no or mild MS only,
- Degenerative MS (annulus to leaflet tip): important to assess calcification, planimetry and PHT not useful
- Follow-up: moderate 3-5yrs, severe 1-2yrs, extremely severe 1yearly
- Previous guidelines: Nisiumura RA et al Circulation 2008, based on mean gradient (moderate: 5-10), PASP (moderate: 30-50), and valve area (moderate: 1.0-1.5)
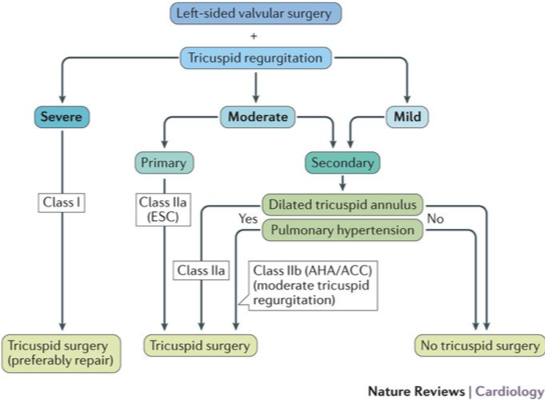
G Scalia – tricuspid valve assessment in the era of structural interventions
- Structure:
- 3 leaflets: AL>SL>PL (size largest to smallest)
- Adjacent anatomy:
- Antero-septal commissure: aortic root
- Anterior: RVOT
- Septal: septum and AV node
- Posterior: RV free wall
- Incomplete fibrotic annulus
- 2D Imaging
- PLAX RV inflow: anterior / posterior
- PSAX: septal / anterior
- SC4C: anterior / septal
- Mechanisms of classification:
- Primary (20%): valvular aetiology
- Functional (80%): annular dilatation, leaflet tethering, RV remodelling, pressure and volume overload
- Annular dilatation occurs in AP direction
- Annulus dilatation and distortion > leaflet malcoaptation
- Severe functional TR increases morbidity (oedema, ascites, anorexia) and mortality
- Carpentier (functional) classification (as per MV)
- Tx difficult: Prolonged recovery, complex anatomy, fragile tissues. If concurrent Lt-sided valve surgery severe TR class I indication, moderate primary TR OR moderate secondary TR with dilated tricuspid annulus or PHTN Class IIa indication
- Tx percutaneous: mitraclip (anterior-septal leaflets, results severe > mild-moderate severity), tricuspid annular reduction (trichinch device, risk tamponade OR trialign device SCOUT trial result 6min walk test 22% improvement and subjectively 62% symptomatic improvement), FORMA spacer (creates platform for native valve coaptation), bi-caval valve implantation, tricuspid cardioband
- Tx surgery (role in other surgery): TVR mechanical (high INR), xenograft, flexible annuloplasty, De Vega procedure
P Pibarot – echo assessment of patients with multiple / mixed valvular diseases
| Combination of valve lesions | Aortic stenosis | Aortic regurgitation | Mitral stenosis | Mitral regurgitation |
| Aortic stenosis | PHT unreliable | PHT unreliable
LFLG MS can occur |
||
| Aortic regurgitation | Simplified Bernoulli equation might not be applicable
Gorlin formula invalid Continuity equation is applicable Peak aortic jet velocity reflects severity of AS and AR |
Aortic regurgitant jet can be mistaken for MS jet
Continuity equation unreliable |
Doppler volumetric method unreliable | |
| Mitral stenosis | LFLG AS is common | MS can blunt increase in PP associated with AR | Not affected | |
| Mitral regurgtiation | LFLG AS is common
MR jet should not be mistaken for AS jet |
Doppler volumetric method inapplicable
PHT unreliable |
Continuity equation unreliable
PHT unreliable Gorlin formula invalid |
- Step1: Number valvular lesions (TTE) > 2. Presence of symptoms and determine if valvular lesions are cause of symptoms (exercise testing, ECG, ESE, BNP) > 3. Repercussion of valvular lesions on cardiac chamber function > 4. Indication and type, timing of valve procedures
- Moderate AS and AR: Moderate AS and moderate AR has similar prognosis as pure severe AS. Peak aortic jet velocity and mean gradient are good markers of overall severity of MAVD.
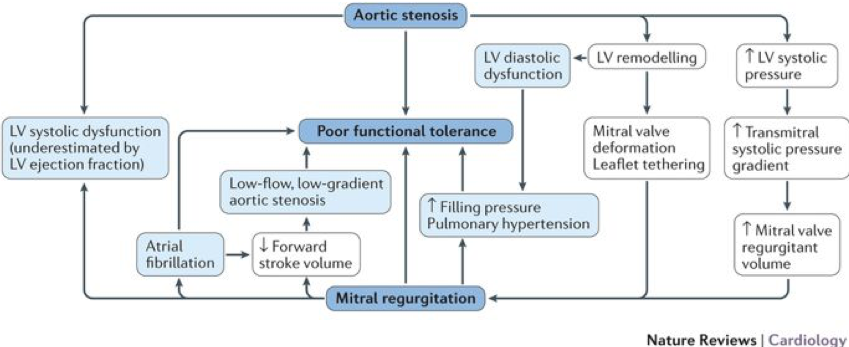
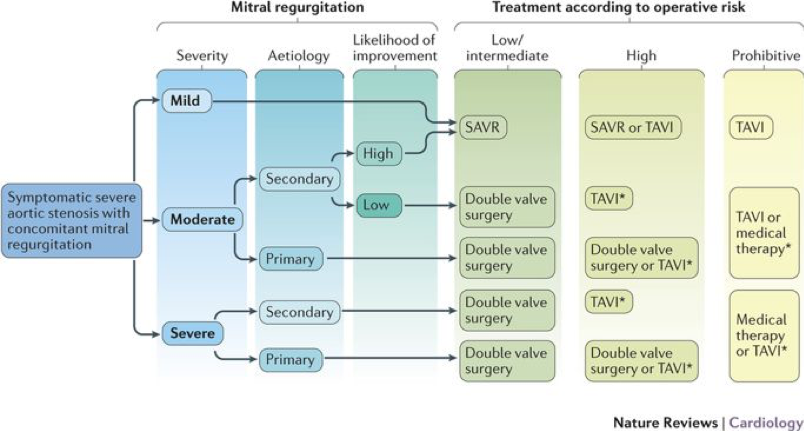
- AS with concomitant MR: may over-estimate EF (due to MR), may over-estimate MR (due to increased LVP secondary to AS), may under-estimate AS (due to reduced forward flow and low-flow state). Low-dose DSE may be used to differentiate true vs pseudo severe stenosis but results often inconclusive. Often MDCT is useful to confirm stenosis.
A Almeida – surgical treatment for functional mitral regurgitation: what can be done?
- Functional MR: secondary to abnormal LV function (regional or general, ischaemic, CM or other) with normal leaflets and cords, leading to mismatch between MV apparatus, which causes increased cord stress, initially leading to leaflet restriction and later rupture (flail leaflet may be due to cord stress)
- A mismatch between ventricular dilatation and MR severity – may be explained by direction of papillary muscle pull, and inter-individual variability regarding amount of leaflet tissue
- Remember preload dependency (caution may under-estimate MR in fasted patient)
- Repair vs replacement severe ischaemic MR:
- 2yr follow-up no mortality difference or change in LV SV between groups, but recurrence of moderate-severe MR 59% in repair group, versus 3.8% in replacement group
- Predictors of favourable outcome for annuloplasty – LV<65mm and likely to recover:
- Echo: tenting height >1cm, tenting area >2,5cm, posterior leaflet angle >45 degrees, distal anterior leaflet angle >25 degrees, asymmetric tethering, LVEDD<6.5cm
- MRI: annuloplasty favoured if LV likely to recovery – If on MRI >25% scarring posterior papillary muscle recurrence of MR is 70% at 6 months. Thought because annuloplasty augments tethering can worsen if LV doesn’t ultimately improve
- Predictors for favourable outcome for replacement – LV>65mm or marked tethering or inadequate leaflet tissue
- Alternatives: shaped annuloplasty rings
Session 3: Diastology and Cardiomyopathies
B Anderson – echo assessment of LV diastolic function
- Diastolic function is the ability to fill to adequate EDV to ensure adequate forward SV in systole
- HFpEF: complex clinical syndrome, abnormality of cardiac structure or function that impairs the ability of the heart to fill with blood with normal pressure
- No single measurement that quantifies diastolic dysfunction: four key variables to assess LVDF
- Mitral annular e’
- Lower e’ implies slower LV relaxation
- Septal e’ <7cm/s lateral e’ <10cm/s
- Mitral average E/e’ >14
- As LVFP mitral E velocity increases, annulus e’ ratio decreases, resulting in increased E/e’ ratio
- Septal E/e’ >15 Lateral E/e’ >13
- LA volume index >34ml/m2
- Morphophysiological expression of increased LV filling pressures (dilated LA consistent with chronically elevated LV filling pressure)
- LA measurement area: A2C view optimise for LA (LV may be off-axis)
- TR peak velocity >2.8m/s
- TR reflects PASP in absence of PS or pulmonary disease
- TR velocity >2.8ms considered abnormal
- Contrast enhancement of TR may improve measurement
- PLUS: supplementary parameters suggesting increased LVFPs:
- Ar-A duration: S/D<1, Ard-Ad >30msec
- Positive vasalva
- L-wave >40cm/sec
- LA strain can help reclassify patients with indeterminate studies (Morris DA et al. JACC Cardiovasc Imaging. 2018 Oct;11(10):1405-1415)
- Isovolumetric relaxation time (IVRT) Ac to MVo NR 70-90ms
- Deceleration time (DT) peak E to zero velocity NR 150-250ms (competing variables: abnormal relaxation lengthens DT, rising LVEFP shortens DT)
- Mitral annular e’
- Two TTE algorithms: depending on LVEF and suspicion of myocardial disease (Anderson OS et al. J Am Coll Cardiol 2017;69:1937-48 / Balaney B et al. J AM Soc Echocardiogr 2018;31:79-88)
- Normal LVEF and no clear myocardial disease: Remember ‘normal’ diastology parameters derived from elderly population (>6pyrs)
- Normal diastolic filling pressures: None or 1 variables fulfilled
- Indeterminate: 2 variables fulfilled, consider using supplementary parameters to reclassify
- Elevated diastolic filling pressures: 3 or 4 variables fulfilled
- Reduced LVEF or normal LVEF plus established diastolic dysfunction or myocardial disease (based on 2016 ASE/EACVI guidelines for estimation filling pressures)
- Normal LVEF and no clear myocardial disease: Remember ‘normal’ diastology parameters derived from elderly population (>6pyrs)
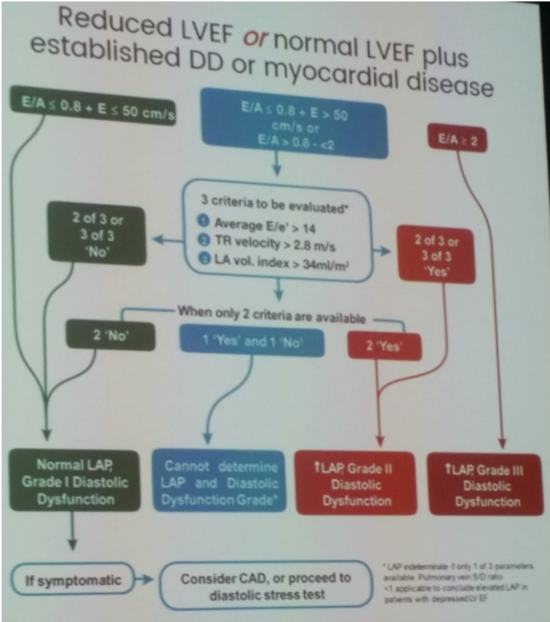
- Indeterminate studies may result from: missing measurements, measurement errors, incorrect usage of algorithm, ignoring clinical presentation or 2D findings, or because of patient exclusions (see below)
D Burstow – LV diastolic function in “special populations”
- Standard diastology parameters affected in conditions that alter mitral inflow velocities:
- AF, paced rhythm, LBBB, mitral valve disease, sinus tachycardia, HCM, MR
- Greater reliance on supplementary parameters to assess diastology in each condition
M Bremer – echocardiography in the evaluation of hypertrophic cardiomyopathy
- Inherited autosomal dominant disease in genes encoding contractile proteins, resulting in LVH in absence of other causes
- Pathophysiology: combination of myocardial ischaemia, autonomic dysfunction, LVOT obstruction and diastolic dysfunction
- Multiple variants of hypertrophic cardiomyopathy (HCM):
- Concentric HCM
- Mid cavity obstruction may lead to ballooning of apex (thinnest part of LV > increased wall stress), subsequent fibrosis and origin for VT/arrhythmias
- HCM with septal hypertrophy and SAM
- Asymmetric hypertrophy (typically of septum), diastolic LV dysfunction, LA enlargement (secondary to DD or MR from SAM of anterior leaflet of MV)
- Different variants (neutral, sigmoid, reverse curvature)
- Apical HCM:
- ECG: large TWI, typically lateral praecordial leads and inferior limb leads
- Different variants (apical HCM, apical HCM with out-pouching, apical HCM with aneurysm), contrast can help identify out-pouching, aneurysm and thrombus
- Echocardiography assessment of HCM: important variables
- Identify presence and distribution of hypertrophy: using 2D and strain
- Strain imaging: thickest wall segments produce most abnormal strain segments
- 70% of HCM patients have obstructive picture, 40% of which are latent obstruction (i.e. dynamic), assess using CWD
- Dynamic LVOT obstruction at rest and with provocation: caution against risk of cavity obliteration
- If significant rest obstruction (resting gradient >50mmHg) no further steps required, if <50mmHg:
- PVC – luck, as greater preload post-ectopic beat, if nil:
- Trial vasalava (unreliable but specific depending on patient performance, high false negative) if >50mmHg on CWD evidence of obstruction, no further steps required, if <50mmHg:
- Trial amyl nitrite, if <50mmHg:
- Consider stress (tough to acquire) or cardiac catheter lab
- Note LVOT and MR can be difficult to distinguish
- Assess MV and papillary muscles: presence or absence of SAM and degree of MR
- Assess LV diastolic function: can be challenging
- Assess LVEF: EF often normal however SV and CO typically impaired
- Assess RV hypertrophy / RVOT obstruction / PASP: prognostic indications
- Identify presence and distribution of hypertrophy: using 2D and strain
- HCM Tx: symptomatic improvement
- Medical: decrease myocardial contractility or change loading conditions
- Surgical: surgical septal myectomy +/- MV repair, alcohol septal ablation, dual chamber pacing (induce dyssynchrony)
- Concentric HCM
D Platts – diagnosis and assessment of dilated cardiomyopathy
- CM=heterogenous group myocardial diseases with mechanical or electrical dysfunction, usually with inappropriate ventricular hypertrophy/dilatation
- Different arbitrary classification systems of CMs:
- AHA (North American system): primary vs secondary CMs
- ESC (European): morphology and function
- MOGES classification system: complicated ?research application
- Dilated CM criteria:
- Dilated LV
- Systolic dysfunction
- Absence of significant CAD / abnormal loading
- Dilated CM is not a single disease entity, but an end stage non-specific phenotype from a broad range of myocardial insults and varying pathogenesis
- Commonest symptom is breathlessness
- Commonest cause of cardiomyopathies and third commonest cause of HF in adults
- Aetiologies: 50% primary and secondary causes, approximately 50% idiopathic (?genetic)
- Algorithm for unexplained CM workup (Bozkurt et al, Circulation 2016;134:e579-e646)
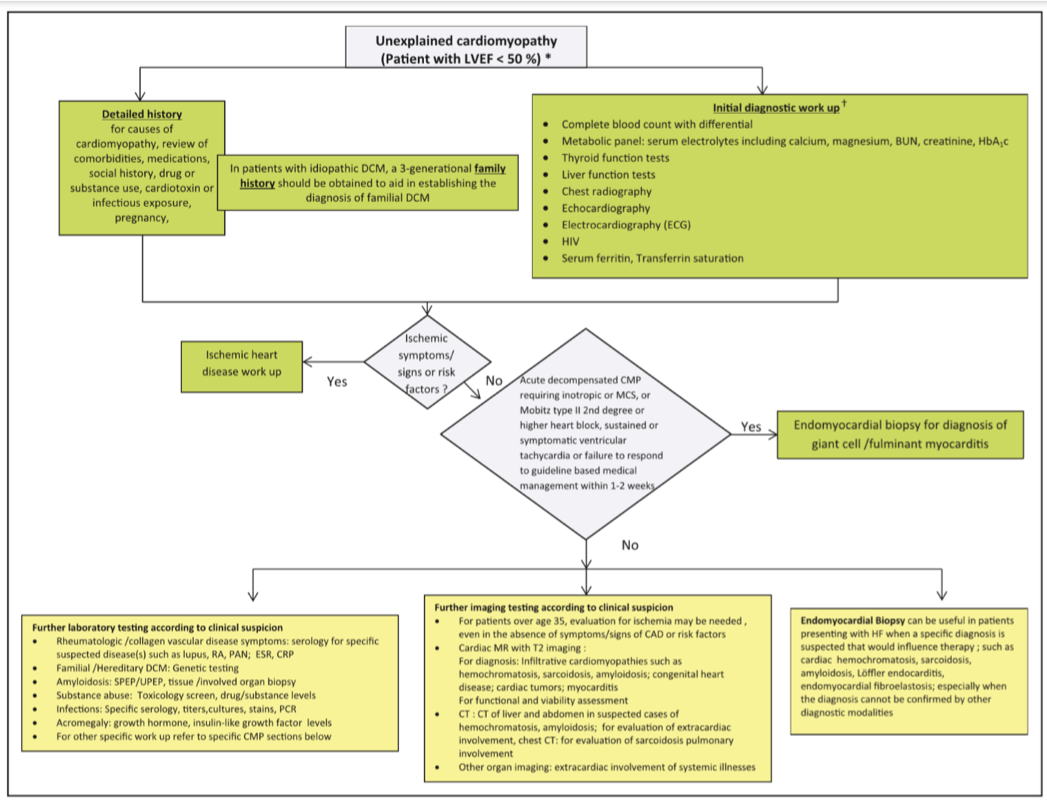
- Ventricular thrombus: DE-cMRI if no thrombus seen on CE-TTE but suspicion persists
- cMRI: myocardial fibrosis predictor of mortality in dilated CM
- Endomyocardial biopsy: new onset HF with non-responsive therapy or eosinophilic concerns
- Genetic inheritance: autosomal dominant, X-linked recessive or mitochondrial inheritance of protein encoding genes
- Hx Familial DCM: 2 or more relatives or SCD <35yrs age
- Clinically evaluate 1st degree relatives (ECG, TTE +/- arrhythmia monitoring)
- Pre-DCM phenotype: LV enlargement without systolic impairment, as a precursor to familial DCM > 25% will have LVE, 10-20% will develop DCM
- If mutation identified, cascade screening throughout family (require genetic counsellors)
J Lindner – the future of echo: molecular imaging and ultrasound therapy
- Belcik JT, et al. Circulation 2017; 135:124 – shear mediated vasodilation resolves tissue ischaemia
Session 4: Pulmonary Hypertension and Diseases of the Aorta
R Cordina – evaluation of the right heart in pulmonary hypertension
- PHTN formal Dx cardiac catheterisation
- Echocardiography is useful for
- Screening
- Serial follow-up
- Prognostication:
- Presence of pericardial effusion reflects high RAP and RV decompensation (impaired venous and lymphatic drainage into RA due to elevated pressure, leading to the development of a pericardial effusion)
- Monitoring ventriculo-arterial relationship
- Ventriculo-arterial uncoupling = ventricle unable to maintain contractility against increased afterload, leading to dilatation, reduced contractility and raised filling pressure
- Laplace’s law: wall thickness protective, hypertrophy often first sign of increased RV overload (measured SC view diastole RV free wall)
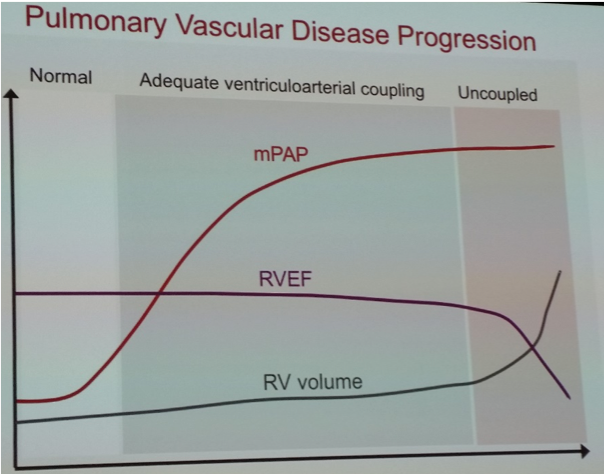
- RV systolic function difficult to assess due to geometry:
- Fractional area change – abnormal <35%
- TAPSE –RV contraction predominantly longitudinal, but often relative sparing at base of heart
- 3D echo – more accurate volumetric assessment, limited by adequate images (lung disease, obesity)
- Strain – evolving area, most important type of strain and normal values not well established
- RV diastolic function:
- RAP – IVC, dilated RA, atrial septum bulging to left
- Interventricular septum – diastolic and/or systolic movement
- Hepatic venous flow pattern – diastolic predominance suggestive RV DD
- E/E’ – lateral annulus (>4 dysfunction)
- Systolic to diastolic duration ratio – measure of global ventricular performance
G Scalia – can echo replace the right heart catheter?
- Normal measurements on Rt heart balloon catheter:
- RAP
- RVP
- PAPsys 30mmHg, PAPdiast 15mmHg, PAPmean <25mmHg
- PCWP (LAP surrogate) <15mmHg
- Trans-pulmonary gradient (TPG) = PAPmean – PCWP <12mmHg
- Diastolic pressure gradient (DPG) = PAPdiast-PCWP
- Pulmonary vascular resistance PCR =TPG/CO <3 wood units
- Echocardiography assessment
- RAP: IVC size and collapsibility (2.1cm / 50%) estimated 3mmHg/8mmHg/15mmHg
- RVSP: TRVmax, ‘chin not the beard’
- LAP: E/e’
- ePLAR: TRVmax/(Mitral E/e’) as means to estimate TPG or pre/post-capillary PHTN
- Pulmonary hypertension: multiple causes, can be divided into arterial or venous hypertension (J Am Coll Cardiol. 2015;65(18)1976-1997), important to distinguish on echo
- Pulmonary venous hypertension (previously known as post-capillary PHTN)
- More common
- Obstructed venous efflux from lungs e.g. LHF
- High LAP, high PAP, normal TPG
- Pulmonary vasodilators won’t help
- Pulmonary arterial hypertension (previously known as pre-capillary PHTN)
- Less common
- Sclerosis and hardening of pulmonary arterial bed, more common young females
- High PAP, low LAP, high TPG (>12mmHg)
- Patients require pulmonary vasodilators
- Pulmonary venous hypertension (previously known as post-capillary PHTN)
H Thomson – aortopathies: transthoracic versus transoesophageal echo
- Aorta segmentation: aortic root > ascending aorta > descending thoracic aorta > suprarenal abdominal aorta > infra-renal abdominal aorta
- Acute aortic syndromes can be complex, Stanford classification; TypeA: ascending aorta and proximal to, mortality 25% in 24hrs (1%/hr), acute presentation, TypeB: distal to ascending aorta
- Transthoracic versus transoesophageal echo, consideration CTA
- TTE – helpful but not always complete
- PSLAX measure trans-sinus, sinotubular, ascending aorta
- RSE to assess arches and further up ascending aorta
- Blind spots: distal descending thoracic aorta and distal ascending aorta
- TOE – more comprehensive, limited by invasiveness
- Blind spot: distal to ascending aorta (due to trachea)
- Normal aorta sizes dependent on age, gender and body size (Goldstein S et al multimodality imaging of diseases of the aorta 2015 28(2);119)
- Inherited aortopathies: syndromic and non-syndrome
- Syndromic:
- Marfan’s syndrome – 1/5000 people but account for 5% all dissections, FBN-1 mutation > excess TGFbeta, Ghent criteria for Dx, particular phenotype, require TTE and CT or MRI to confirm size and examine distal ascending aorta, dilatation of sinuses but often preservation of sinotubular dilatation until late
- Loieys-Dietz syndrome – specific phenotype, TTE and CT/MRI of entire vasculature from pelvis to cerebrum as can affect all blood vessels, aneurysms can rupture at smaller size than Marfan’s
- Turner’s syndrome
- Non-syndromic:
- Familial TAA syndrome – genetic related, phenotypically normal individuals, imaged as per Marfan’s, screen 1st degree relatives as often autosomal dominant inheritance
- Bicuspid aortopathy – often lose sinotubular junction, 1% population, male predominance, risk aortopathies, almost all need surgery within lifetime, require TTE and CT including cerebral vessels as is association with cerebral aneurysms, 1st degree relatives require TTE screen
- Cardiac society guidelines (North American and European differ) for intervention
- Syndromic:
- TTE – helpful but not always complete
Session 5: Pericardial Disease
D Burstow – pathophysiology of compressive pericardial disease
- Compressive pericardial diseases; 3 types:
- Tamponade – fluid compression
- Effusive-restrictive pericarditis – fluid plus thickened, stiff pericardium
- Constrictive pericarditis – thickened, stiff pericardium
- DDx: restrictive myocardial disease (important to distinguish)
- Pathophysiology:
- Elevated and equalised central venous, pulmonary venous and ventricular diastolic pressures
- Dissociation (loss of normal respiratory tracking) between intra-thoracic and intra-cardiac pressures:
- Normally negative transmural filling pressure (intra-cardiac to intra-thoracic pressure)
- Intra-cardiac pressure – difference between LAP (estimated by PACWP) and intra-pericardial pressure
- Intra-thoracic pressure – estimated by pulmonary venous pressure, as intra-thoracic but extra-pericardial
- In normal physiology, reduction in intra-cardiac and intra-thoracic pressures to same degree during inspiration
- In compressive pericardial diseases, dissociation between intra-cardiac and intra-thoracic pressures:
- During inspiration intra-cardiac pressure falls less because of constrictive pericardial barrier
- Subsequent reduction in effective filling gradient of LV
- Consequently reducing ventricular filling and ultimately CO
- Normally negative transmural filling pressure (intra-cardiac to intra-thoracic pressure)
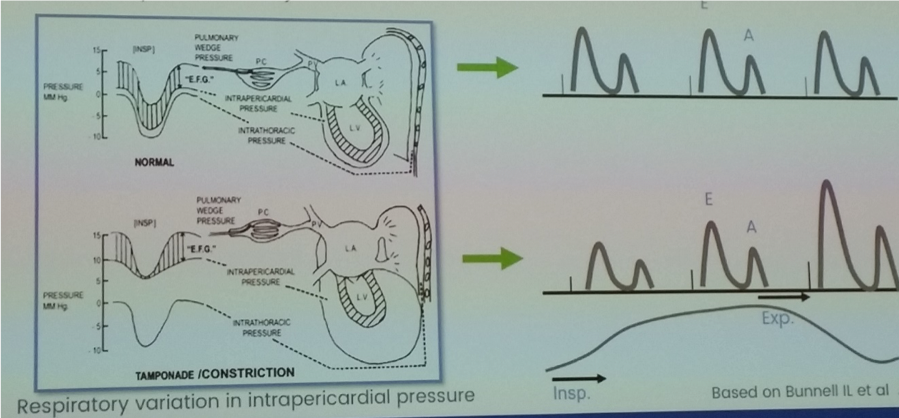
- Ventricular interdependence: fixed total intrapericardial volume leading to increased ventricular interaction as competing for space within the heart
- Septal bounce
- Respiro-phasic variation in ventricular filling (doppler TV, MV and HVs)
- Ventricular filling: physiology differs between tamponade (progressive pan-diastolic loss of filling) verses constrictive pericarditis (initially filling vigorous, then further filling limited by non-compliant myocardium as manifested in rapid mitral E velocity deceleration time)
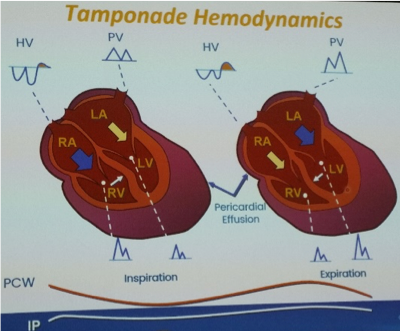
M Bremer – echo diagnosis of cardiac tamponade
- Tamponade occurs when heart cannot dilate sufficiently to receive blood (diastolic problem)
- Accumulation of pericardial fluid (rate and volume)
- Limitation of ventricular filling / chamber collapse
- Reduction of CO (specifically SV)
- Clinically: tachycardia, elevated JVP, pulsus paradoxus, hypotension, diminished heart sounds
- Compensatory: hyperdynamic systolic function, tachycardia, peripheral vasoconstriction, elevation of filling pressures, pericardial stretch (relatively elastic but slow)
- Echo: utilise 2D, M-mode and doppler features:
- Pericardial effusion – differentiate from pleural localisation to DTA
- Swinging heart – may be ECG electrical alternans
- Chamber collapse – RV (diastolic) and RA (systolic) chamber collapse, M-mode to differentiate chamber collapse from contraction
- IVC plethora – >90% patients with tamponade
- Ventricular interdependence – respiro-phasic changes include septal shift and doppler variation, why important to use respirometer with adequate sweep speed and gain (note, opposite for patients on PPV)
- Septal shift and ventricle size changes: IVS Lt>Rt and LV filling increases during expiration (RV decreases), IVS Rt>Lt and RV filling increases during inspiration (LV decreases), M-mode may assist assess LV and RV size, and PSAX outflow to assess variation RV size
- Doppler: assess mitral and tricuspid inflow velocities and hepatic vein (HV) flow reversal, note changes may be present in varying degrees and high filling pressures may mask respiratory changes
- MV flow pattern >25% respiratory-phasic variation – flow increases dramatically during expiration (assess velocity on first QRS complex after onset of expiration) and decreases on inspiration (assess velocity on first QRS complex after onset of inspiration)
- TV flow pattern >40% respiratory-phasic variation – flow increases dramatically during inspiration and decreases on expiration (note 40% variation as normally some degree of variation across TV)
- Expiratory HV end-diastolic flow reversal (+ increased inspiratory hepatic forward flow)
- Tx: pericardiocentesis
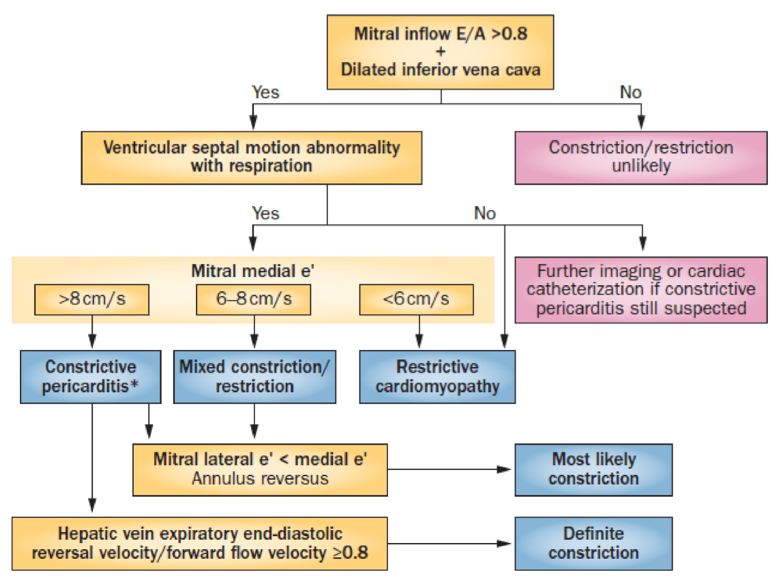
A Luis – constrictive pericarditis verses restrictive cardiomyopathy
- Constrictive pericarditis: idiopathic, post CTHR, radiation, connective tissue, infectious, other including acute pericardial inflammation causing restrictive physiology
- Thickening of pericardium
- Diastolic ventricular filling and distention limited
- Rapid early diastolic filling with small atrial contribution
- Restrictive cardiomyopathy: idiopathic, infiltrative, storage, systemic, chemotherapy
- Thickened stiff myocardium
- Impaired diastolic function
- Must differentiate constrictive pericardial from restrictive myocardial physiology:
- Both similar clinical presentations, both produce severe biventricular HF (non-dilated, often preserved LV systolic function, bi-atrial dilatation), but different Mx

- Both similar clinical presentations, both produce severe biventricular HF (non-dilated, often preserved LV systolic function, bi-atrial dilatation), but different Mx
- Echo to detect and differentiate:
- Mitral inflow E/A >0.8 + IVC plethora
- Ventricular interdependence: septal motion abnormality and ventricle size changes
- Ventricular interdependent: doppler parameters
- Tissue doppler imaging (TDI): measures myocardial tissue velocity to assess relaxation, early diastolic mitral annular velocity (e’) reflects LV relaxation, normally >8cm/sec medial mitral annulus
- Constrictive physiology:
- Lateral annulus motion limited by tethering to pericardium therefore low lateral mitral valve annulus TDI,
- Increased medial TDI as not tethered and increased movement to compensate for lateral annulus,
- Produces annulus reversus where medial TDI > lateral TDI
- Restrictive – loss of longitudinal LV mechanics with low medial and lateral MA TDI, lateral TDI > medial TDI
- Constrictive physiology:
- Valvular inflow doppler: marked respiro-phasic valvular inflow changes in both constrictive and restrictive physiology (MV>25%, TV>40%)
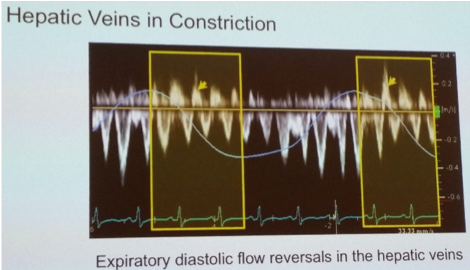
- Hepatic veins: expiratory hepatic venous end-diastolic flow reversal (constriction), versus inspiratory hepatic venous diastolic flow reversal (restriction)
- Tissue doppler imaging (TDI): measures myocardial tissue velocity to assess relaxation, early diastolic mitral annular velocity (e’) reflects LV relaxation, normally >8cm/sec medial mitral annulus
- Supportive information:
- Global longitudinal strain: regionally reduced strain in lateral tethered segments (constriction), global non-specific pattern of GLS reduction (restriction)
- Note annulus reversus AND expiratory HV end-diastolic flow reversal both possible in obstructive airways disease (particularly young patients due to exaggerated inspiratory efforts): loss of respire-phasic variation of systolic wave also suggests constrictive disease
Session 6: Ischaemic Heart Disease and Stress Echo
J Chan – stress and strain: is it really worth the effort
- Stress echo by RWMA is a qualitative technique, affected by human factor (inter-observer variation even at expert level) and LV tethering
- Combination of RWMA and strain improves sensitivity and specificity for detection of ischaemia and viability – strain imaging does not replace RWMA but used in conjunction
- Strain imaging also potential for identification of subclinical ischaemic CM
J Humphries – complications of MI: nightmare cases
- Papillary muscle rupture
- ~1% post MI (typically inferior)
- 24hrs with thrombolysis, 2-7 days without thrombolysis
- Posteromedial papillary most commonly affected
- Echo: mobile masses, chaotic cord motion, flail MV, acute severe MR, V-cut off sign, small hyperdynamic LV, often no RWMA (often small distal artery involved), usually normal LA size, use off-axis imaging to view sub-valvular apparatus
- Free wall rupture
- Most common site lateral LV wall (50% within 5-days, 90% within 2/52)
- Rupture and death from haemopericardium and tamponade
- Pseudoaneurysm – contained rupture, narrow neck, globular in shape, to-and-fro between PA and LV (CFD), high rupture risk, UEA can aid diagnosis, must differentiate from aneurysm (true aneurysm secondary to myocardial weakening, wide neck, rare rupture risk)
- Most common site lateral LV wall (50% within 5-days, 90% within 2/52)
- Ventricular septal rupture
- ~1% post MI
- 24-28hrs post reperfusion, 3-5 days without reperfusion, rare after 2 weeks
- Simple: direct defect, usually apical secondary to inferior MI, important to tilt posteriorly A4C to assess
- Complex: serpiginous course through IVS, usually multiple, interrogate areas of dyskinesis with CFI use multiple views including off axis
- Can estimate RVSP from peak VSR velocity,
- SBP=RVSP+VSRpeak, therefore RVSP=SBP-VSRpeak
J Lindner – LV systolic function: new methods rooted in old physiology
- Different fibre orientation throughout heart
- Cardiac work = HR x stroke work (where stroke work = area within PV relationship)
- Contextualise echo findings based on clinical scenario and basic physiology (e.g. increased afterload from SBP decreasing strain and SV)
P Pibarot – role of stress echocardiography in non-ischaemic heart disease
- Valvular heart disease: valve lesions are dynamic but often only assessed statically
- Stress echocardiography may reveal dynamic component to the valvular lesion, consider if mismatch between valve lesion and symptom severity (i.e. severe lesion and asymptomatic, or symptomatic with statically not significant valve lesion)
- ESE in asymptomatic aortic stenosis (contra-indicated in severe Sx AS), AVR recommendations:
- Class I – asymptomatic severe AS and abnormal exercise symptoms (1/3rd of ‘asymptomatic’ individuals will have exercise limiting Sx)
- Class IIa – asymptomatic severe AS and abnormal exercise
- Class IIb – asymptomatic severe AS with increase in gradient >20mmHg during exercise
- DSE in classical symptomatic LFLG AS (LVEF <50%, AVA <1cm2, MG<40mmHg), to assess MPG and AVA to grade AS severity and guide AVR recommendations:
- Class I – LFLG AS with severe AS with flow reserve on DSE
- Class IIa – LFLG AS with severe AS without flow-reserve on DSR
- Class IIb – LFLG AS with DSE MG>40mmHg
- ESE in MS, recommendations for PBMV:
- Class I – symptomatic severe MS
- Class IIa – asymptomatic very severe MS
- Class IIb – asymptomatic mod-to-severe MS and haemodnymically significant MS during exercise (MG>15mmHg, systolic PAP>60mmHg)
- SE in asymptomatic severe MR
- Class I – asymptomatic severe MR and symptoms on exercise
- Class IIb – pulmonary HTN on exercise (systolic PAP>60mmHg)
- Predicting persistent LV dysfunction post-operatively may be assessed by GLS
- ESE in asymptomatic aortic stenosis (contra-indicated in severe Sx AS), AVR recommendations:
Masterclass: Sonographer Basics
B Anderson – strain imaging (speckle tracking)
- Strain should be routine measurement; most common clinical application is GLS
- GLS assessed in APLAX / A4C / AP2C and displayed x4 format or Bull’s eye plot (17 segment model), red (negative strain) and blue (positive strain)
- 5 steps for strain calculation (Paper: Research to Practice: Yang et al, JACC; Cardiovascular Imaging 2018)
- View selection
- Tracing
- Assessment of tracking quality
- Defining end-systole (AV closure APLAX view or on AV doppler trace)
- Integration of 3 AP views for LVGLS
- Uses of strain imaging
- Pattern recognition: to determine aetiology
- Subclinical dysfunction: detects early subclinical myocardial dysfunction before LVEF (relative reduction % GLS >15%) e.g. CTx to consider Mx strategy
- Mechanical dispersion (MD): start QRS to peak strain, normally peak simultaneously, vs widening (>65-70ms) for prediction potential ventricular arrhythmias
Masterclass: Sonographer Advanced
M Bremer – mitral regurgitation
- Quantification if: visually moderate or greater, eccentric jet, clinical suspicion, serial assessment
- Antoine et al Circulation 2018;138:1317-1326: EROA strongest indicator of survival in MR
- Quantification methods
- Qualitative or semi-quantitative methods e.g. Vena contracta (<0.3cm / 0.3 – 0.69 / >0.7cm)
- Quantitative volumetric method (if no other significant valvular leak), but, multiple measurements implies multiple potential errors
- Mitral SV = total LV stroke volume + MV regurgitant volume
- MV Regurgitant SV = LV SV (LVOT CSA, VTI LVOT flow) – Mitral SV (MAA CSA x TVI MA flow)
- MV diameter: CSA of mitral annulus during diastole at leaflet insertion into annulus during apical view
- MV VTI: flow at annulus measured by PWD, average of 3 measurements, apical view
- MV Regurgitant fraction (%) = (MV regurgitant volume / MV inflow volume) x100
- Effective regurgitant orifice area (ERO cm2) = regurgitant volume / MR VTI
- Quantitative PISA method (proximal isovelocity surface area) – useful if other valvular lesions present, only 2 measurements (fewer variables>fewer potential errors)
- Concentric shells varying velocity, measure using CFD and Nyquist limit
- Law conservation of mass (flow proportionate on each side where flow = area x velocity)
- Method
- Magnify MV 2D image
- Shift CFD baseline in direction of flow
- MR radius and velocity (CWD) measured mid-late systole
- ERO = flow / velocity
- Regurgitant volume = ERO x TVI
- MR severe (primary and secondary): EROA >0.4cm2 / regurgitant volume >60ml / regurgitant fraction >50%
R Chamberlain – aortic regurgitation
- Qualitative methods and semi-quantitative parameters
- Jet width / LVOT diameter
- Jet area / LVOT area
- Vena contracta width
- PHT
- CW doppler intensity
- Quantification: all AR unless mild
- Why: high morbidity and mortality, 83% of patients have cardiac events by 10years if untreated
- Quantification methods (as for MR):
- Doppler SV method
- PISA method
- Important parameters: EROA / regurgitant volume / regurgitant fraction
S Kay – strain in oncology
- Cardiotoxicity
- Cardio-Oncology: clinical manifestations of effects (multiple potential mechanisms) of oncology agents on the CVS, field evolved from childhood cancer patients since Tx 1979 with anthracyclines, different causative agents now identified:
- Anthracyclines
- Alkylating agents
- Taxans
- Antimetabolites
- Endocrine therapy
- HER2
- Cyclin-dependent kinase 4/6 inhibitors
- Radiation therapy
- Other cardiotoxicity: Psych (schizophrenic, some antidep), neurological (PD), oncology (medications, some cancers), athletes (load endurance training, AVRD, long QT)
- Cardio-Oncology: clinical manifestations of effects (multiple potential mechanisms) of oncology agents on the CVS, field evolved from childhood cancer patients since Tx 1979 with anthracyclines, different causative agents now identified:
- Cardiotoxicity in breast Ca: intersecting RFs of cardiac disease and breast Ca, superimposed cardiotoxicity of breast Ca Tx is therefore an important adverse reaction
- Anthracycline regimes e.g. doxorubicin HF risk – 400mg/m2 5% risk, 550mg/m2 26% risk, 700mg/m2 48% risk
- Radiotherapy – volume of heart within radiation field critical to toxicity, utilise heart sparing techniques (deliver RTx at end of deep inspiration to avoid heart) > reducing mean heart dose radiation
- Current detection methods for HF: Sx and Hx, ECG, EST, gated heart pool scans, TTE, stress Echo, coronary CT, MRI/cMR, strain imaging
- Strain imaging:
- GLS change >15% definite cardiotoxicity
- GLS <8% no evidence of cardiotoxicity
Masterclass: Adult Congenital Heart Disease Basics
B Anderson – tips and tricks: a primer for echo in ACHD
- Segmental approach: start at the apex, draw it out, identify chambers, arteries, AV/VA connections and veins, may be several and multiple types of defects, off axis imaging often key
- Relative atrial index (RAI) for ASDs
- RAI = RA area / LA area
- Cut-off value 0.92 predict patients ASDs 99% sensitivity, 90% specificity
- DDx significant TR, PHTN
- x4 types ASDs (sprimum, secundum, superior and inferior sinus venosus defect, coronary sinus defect)
- RAI = RA area / LA area
- Aorta coarctation: CWD suprasternal notch descending thoracic (saw-toothed pattern) and abdominal aorta (continuous non-pulsatile flow)
- PR severity post ToF repair:
- OT of ToF: Patch closure of BSD and opening of pulmonary trunk
- Post-op PR complication often missed as low-velocity, laminar flow, and short duration
- Chronic severe PR leads to RV dilatation, increased arrhythmia’s, SCD, and if detected late is irreversible
- Echo: CWD across pulmonary valve key (rapid equalisation between PA and RVSPs), diastolic flow reversal in PA branches
J Fawcett – shunt lesions: echo identification and assessment of
- VSD: peri/membranous (most common), muscular, inlet, outlet
- Gerbode defect: LV to RA
- PDA: result in Lt heart volume overload
R Cordina – obstructive lesions: echo identification and assessment of
- Obstructive lesions often occur in series
- Very common in setting unrepair and repaired CHD
- Easy to miss obstructive lesions, examples:
- Venous obstruction: often post repair (e.g. warden repair of anomalous PV connection e.g.2. mustard repair for TOGAs)
- RVOTO: double chambered RV, sub-valvular, valvular, distal MPA branches