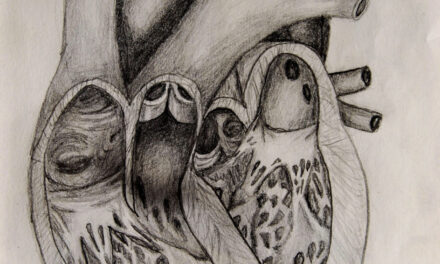
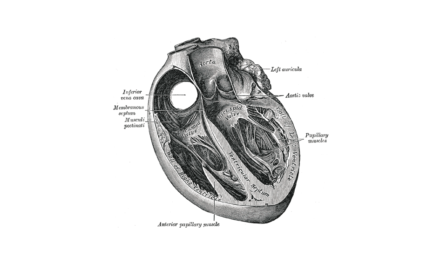
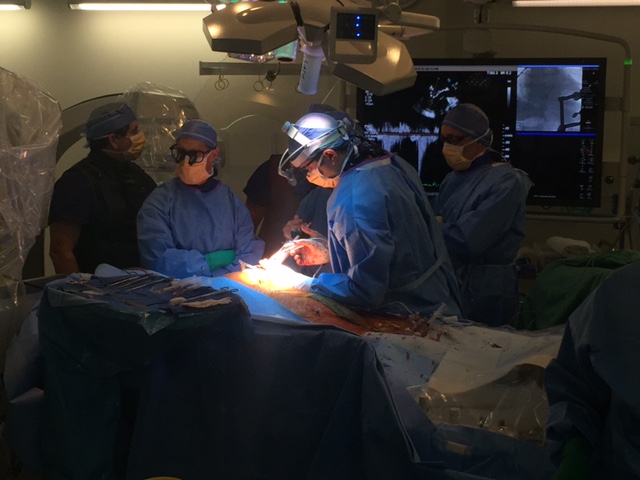
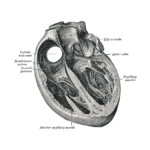
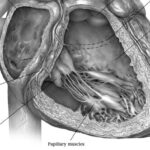
Tendyne Transcatheter Mitral Valve Implantation; viewed by Kim Castledine (St Vincent’s Private Hospital Sydney).
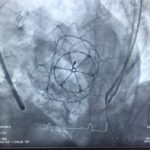
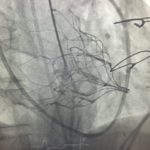
On the 9th of September 2016, the St Vincent’s Hospital Sydney (SVHS) Cardiac Catheter Centre was transformed into a hybrid interventional laboratory/operating theatre to perform two Transcatheter Mitral Valve Implantation (TMVI) procedures. Pioneered by interventional-cardiology specialist, Professor David Muller and renowned cardiothoracic surgeon Paul Jansz in November 2014(3), the TMVI mini-invasive surgical procedure has been utilised for patients who are unsuitable for conventional mitral valve (MV) surgery via sternotomy.
In partnership with Tendyne Holdings, Inc. (Minnesota, U.S.A), clinical trials are being performed at SVHS, using the Tendyne mitral valve device delivery system. This device features an apically tethered tri-leaflet porcine pericardial valve sewn onto a Nitinol frame(1). Three company representatives were in attendance throughout the procedural day and provided essential educational and hands-on technical support to the SVHS staff team.
Patients at SVHS are selected to participate in the Tendyne TMVI clinical study, following a comprehensive screening process co-ordinated by Professor Muller’s research nurses, Erika O’Dea and Alexis Arriagada. Some of the criteria, which deem a candidate as ineligible, include: left ventricular ejection fraction <30%, prior MV or aortic valve surgery, severe coronary disease, heart failure requiring inotropes, severe tricuspid valve regurgitation and severe right ventricular dysfunction(2).
Patient eligibility criteria:
- Severe mitral regurgitation (MR) of primary or secondary aetiology
- New York Heart Association (NYHA) functional Class ³
- Cardiothoracic team to determine patient unsuitable for traditional surgical approach
- Eighteen years or older
Considering Mitral Regurgitation (MR) as one of the leading causes of valvular heart disease (1), patients deemed inoperable for open-heart surgery may benefit from the quality of life improvement that the TMVI approach offers. In addition to increased need for hospitalisation, Severe MR left untreated, can lead to heart failure and five-year mortality >50% (1&3). To date, SVHS has successfully implanted Tendyne mitral valve devices into nine candidates.
TMVI procedure:
- General anaesthetic administered
- Establish access to right and left femoral arteries
- Trans-apical approach via left mini-thoracotomy
- Left ventricular apical puncture
- Advancement of the Tendyne delivery system across MV into left atrium
- Deployment of Tendyne MV under Trans-oesophageal echocardiography guidance
- Tether device sewn into left ventricular apex
- Confirmation of valve position and nil evidence of left ventricular outflow tract obstruction
- Mini-thoracotomy closure
- Femoral arterial sites closed with Perclose devices
Key Benefits:
- Cardiopulmonary bypass not required
- Tendyne TMVI is fully retrievable and repositionable
- Lower rates of post-op complications
- Decreased length of stay in hospital
The post-operative care requirements are likely to vary from patient-to-patient and will involve the continued collaboration of staff members from multi-disciplinary teams. Follow-up guidelines are in place as per the clinical trial protocol in order to review the progress of patients enlisted within the study.
Nursing Considerations:
- Intensive Care Unit; initial ventilation assistance and inotrope support (noradrenaline, dobutamine, glyceryl-trinitrate)
- Close monitoring of vital signs, especially blood pressure
- ECG monitoring
- Mini-thoracotomy wound pain; possible patient-controlled analgesia
- Fluid balance recording; urinary catheter
- Pericardial drain management
- Assessment of neurovascular observations and femoral puncture sites
- Day 1 post-operative echocardiogram
- Daily weight
- Warfarin for minimum of 3 months; close monitoring of INR (target 2.5-3.5)
Structural Heart Disease Australia is providing excellent opportunities for clinicians to witness surgical procedures in a range of facilities, which I highly recommended to fellow colleagues.
References:
- Moat N, Duncan A, Lindsay A, Quarto C, Blanke P, Leipsic J, Grayburn P & Davies S 2015, ‘Transcatheter Mitral Valve Replacement for the Treatment of Mitral Regurgitation: In-Hospital Outcomes of an Apically Tethered Device’, J Am Coll Cardiol. 2015; vol. 65 no. 21, pp. 2352-2353.
- Muller D, Jansz P, Shaw M, Bae R, Farivar R, Sorajja P, Sun B & Pedersen W 2015, ‘TCT-51 The Tendyne Transcatheter Mitral Valve Implantation (TMVI) Global Feasibility Trial’ J Am Coll Cardiol. 2015; vol. 66, no. 15_S.
- Tendyne Holdings, Inc. 2016, Tendyne Announces First Enrolment in TMVI Clinical Trial, Tendyne Clinic PR, viewed 10 September 2016 <http://www.tendyne.com/assets/tendyneclinicpr20141215.pdf>