The VIVID (Valve-in-Valve International Data) group have published a standardised set of definitions for structural valve degeneration (SVD) of surgical and transcatheter aortic valve bioprostheses. They also make recommendations for the timing of clinical and imaging follow-up.
Definition
- An acquired intrinsic bioprosthetic valve abnormality defined as deterioration of the leaflets or supporting structures
- Resulting in thickening, calcification, tearing, or disruption of the prosthetic valve materials with eventual associated valve hemodynamic dysfunction
- Is caused by tissue disruption or thickening over time because of mechanical stress + abnormal flow shear stress
- SVD does NOT include patient-prosthesis mismatch, device malposition, paravalvular regurgitation, & abnormal frame expansion
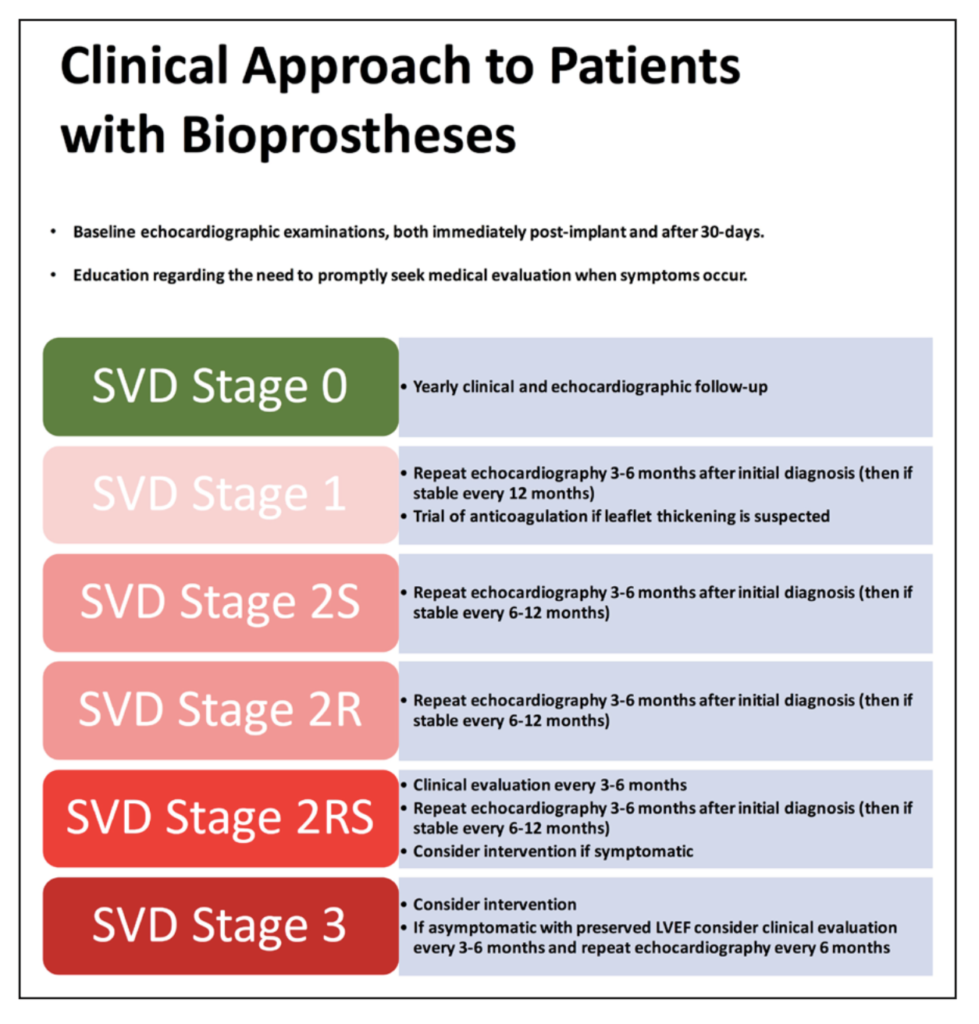
- The process is usually gradual (over years) and so has been divided into stage
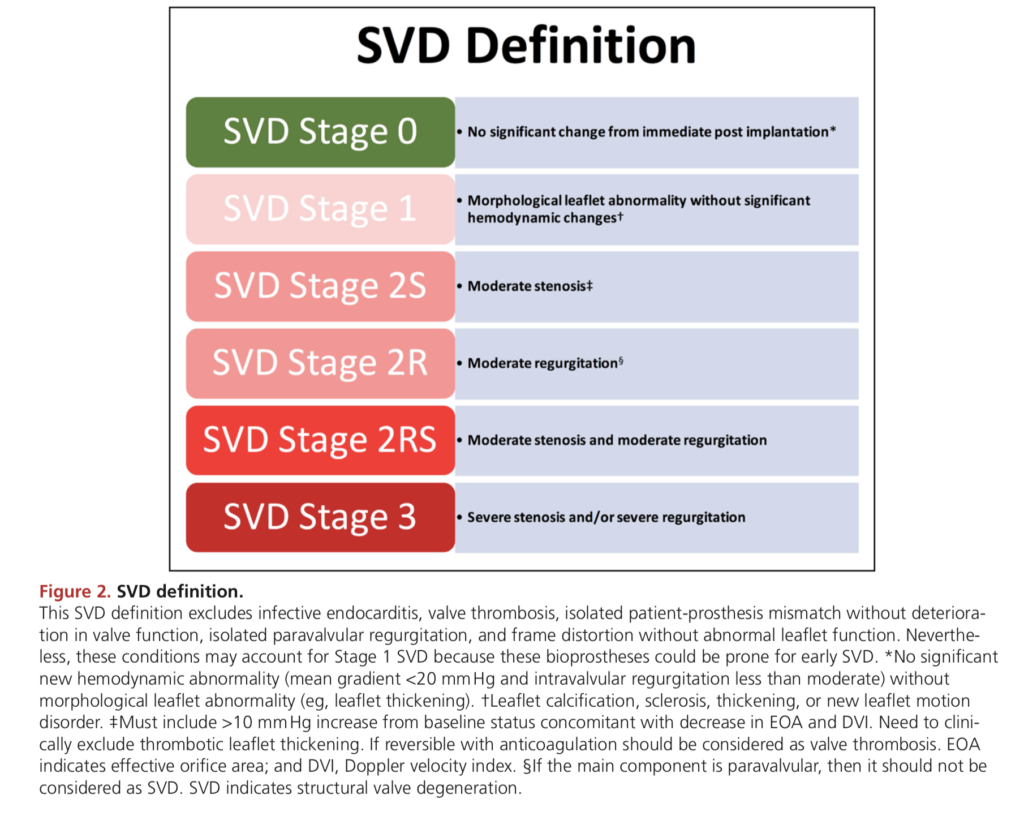
Echocardiographic Assessment
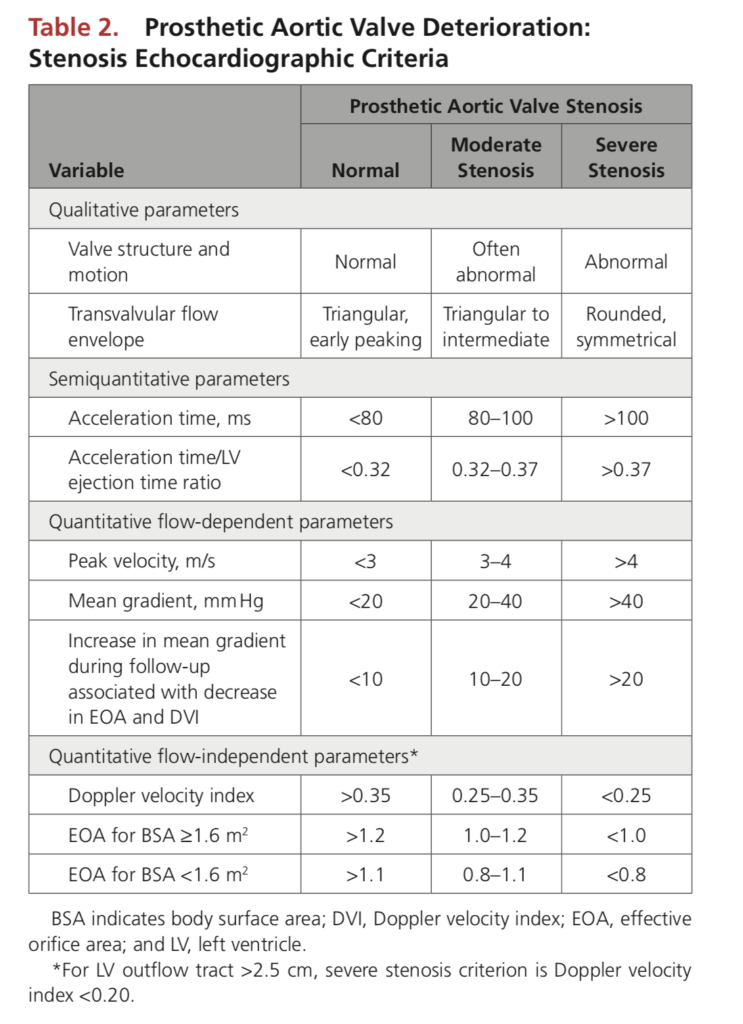
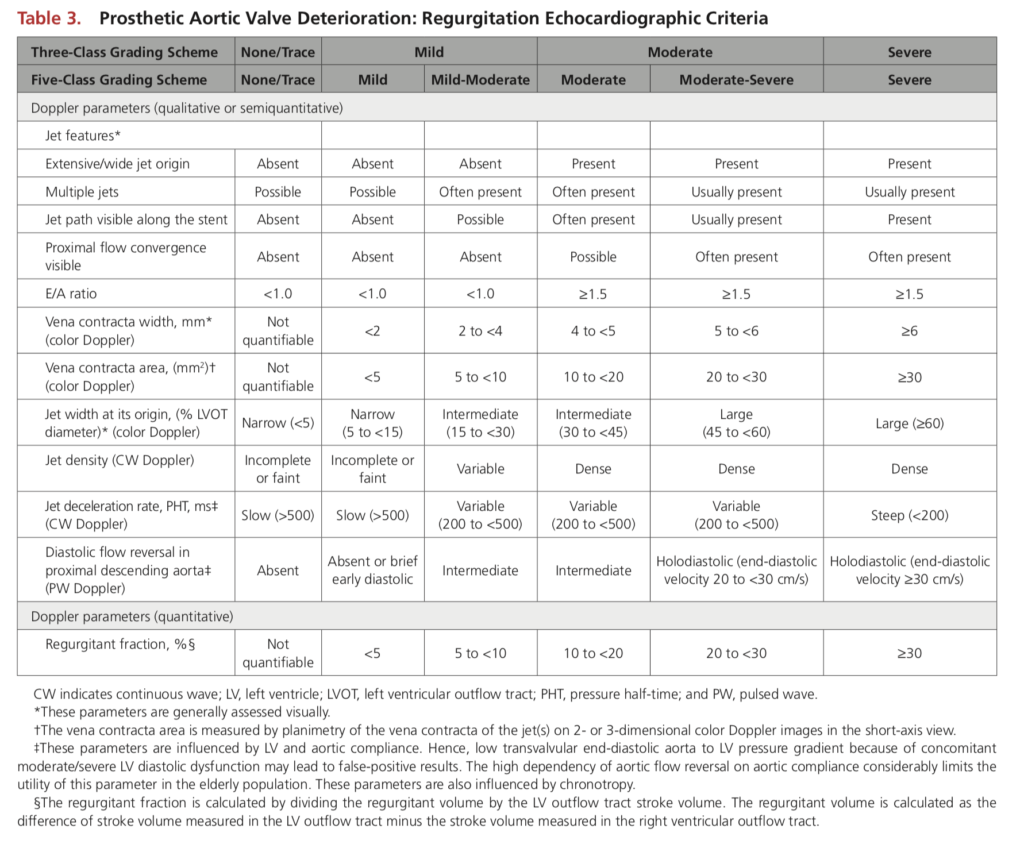
Clinical Application
- Routine post-procedural anticoagulation is currently a IIa recommendation for 3 months post SAVR
- If a change in mean gradient > 10 mmHg or a clinical event (stroke, heart failure, decreased EF, new PVL) then 4D CT or TOE should be done
- If leaflets are thickened should consider a trial of anticoagulation
- All patients diagnosed with SVD should undergo a repeat TTE within 3 to 6 months to evaluate for rapid progression
- For symptomatic patients with severe AR or AS, or moderate mixed AS/AR, should be considered for redo SAVR or valve-in-valve TAVR