Summarised by Andrew Haymet
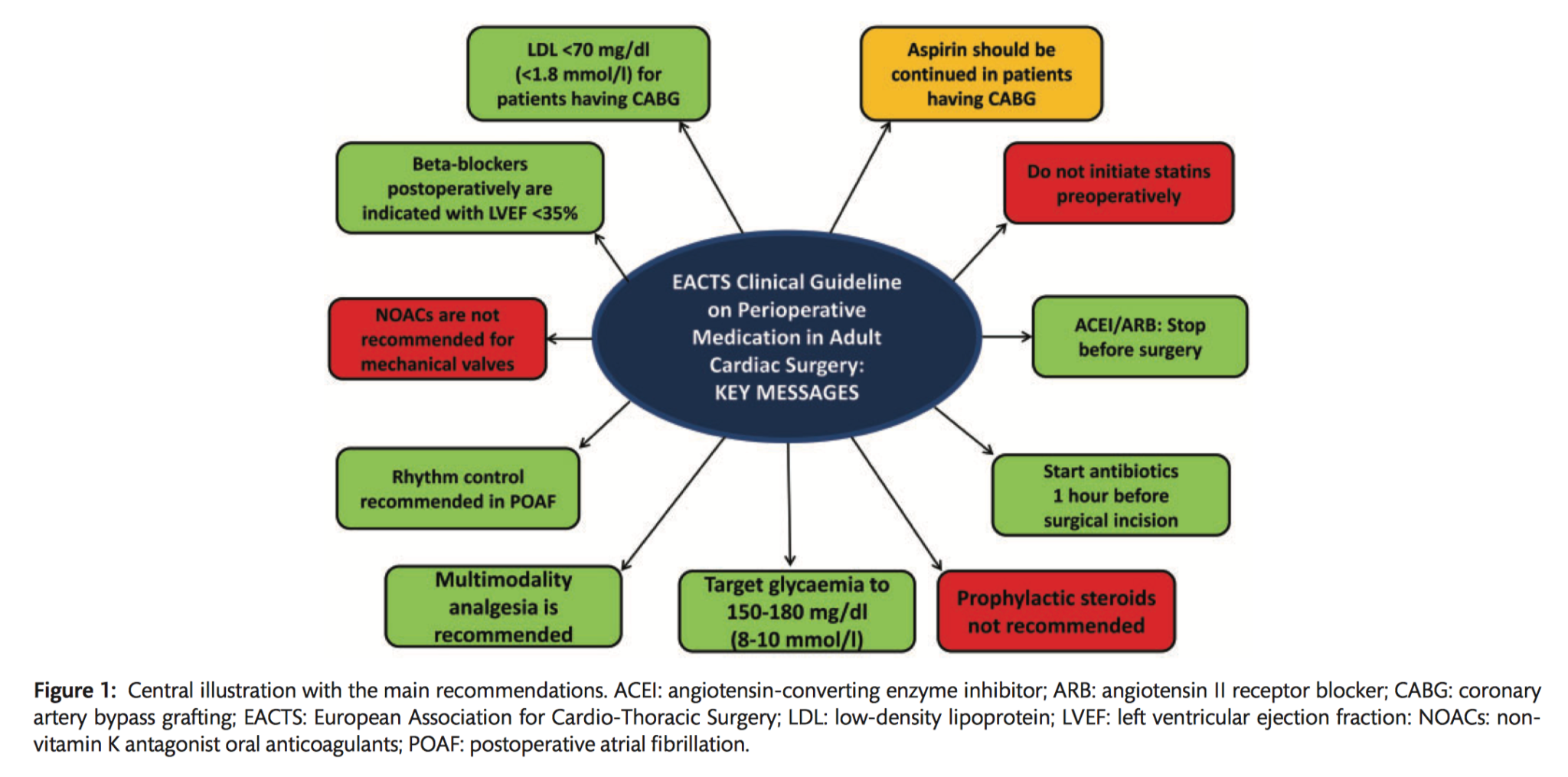
This publication from EACTS aims to provide an updated guideline focusing on the main pharmacological classes involved in the perioperative management of adult patients undergoing cardiac surgery.
Multidisciplinary decision making
- It is recommended that the Heart Team discuss the optimal timing of stopping antithrombotic preoperative treatment of patients undergoing cardiac surgery, based on ischaemic and bleeding risk (Class I C).
Antithrombotic management
Acetylsalicylic acid (ASA)
Preoperatively:
- In patients on ASA who need to undergo CABG surgery, continuing ASA throughout the preoperative period should be considered (Class IIa C).
- Stopping ASA at least 5 days before surgery should be considered in patients who refuse blood transfusions, undergo non-coronary cardiac surgery, or are at high risk of re-exploration for bleeding (Class IIa C).
- Bridging oral antiplatelet therapy with LMWH is not recommended (Class III B).
Postoperatively:
- It is recommended to restart ASA as soon as there is no concern over bleeding, but within 24 hours of CABG (Class I B).
- In patients undergoing non-coronary cardiac surgery with a preoperative indication for ASA, it is recommended that treatment is restarted as soon as there is no concern over bleeding, but within 24 hours of surgery (Class I C).
P2Y12 management
Preoperatively:
- In patients on DAPT who need to undergo non-emergent cardiac surgery, postponing surgery for at least 3 days after discontinuation of ticagrelor, 5 days after clopidogrel, and 7 days after prasugrel should be considered (Class IIa B).
- Platelet function testing may be considered to guide the decision on the timing of cardiac surgery in patients who have recently received P2Y12 inhibitors (Class IIb B).
- Bridging P2Y12 inhibitors with GPIIb/IIIa inhibitors or cangrelor may be considered in high ischaemic risk patients (Class IIb C).
Postoperatively:
- In patients treated with DAPT after recent coronary stent implantation (within 1 month) who subsequently undergo cardiac surgery, it is recommended to resume the P2Y12 inhibitor postoperatively as soon as there is no concern over bleeding but within 48 hours of surgery, and continue DAPT until the recommended duration of therapy is completed (Class IC).
- In patients treated with DAPT after coronary stent implantation (exceeding 1 month) or ACS without stenting who subsequently undergo cardiac surgery, it is recommended to resume the P2Y12 inhibitor postoperatively as soon as there is no concern over bleeding but within 96 hours of surgery, and continue DAPT until the recommended duration of therapy is completed (Class IC).
- DAPT may be considered after CABG in selected patients with stable CAD (endarterectomy, off-pump) (Class IIb C).
Oral anticoagulants
Preoperatively:
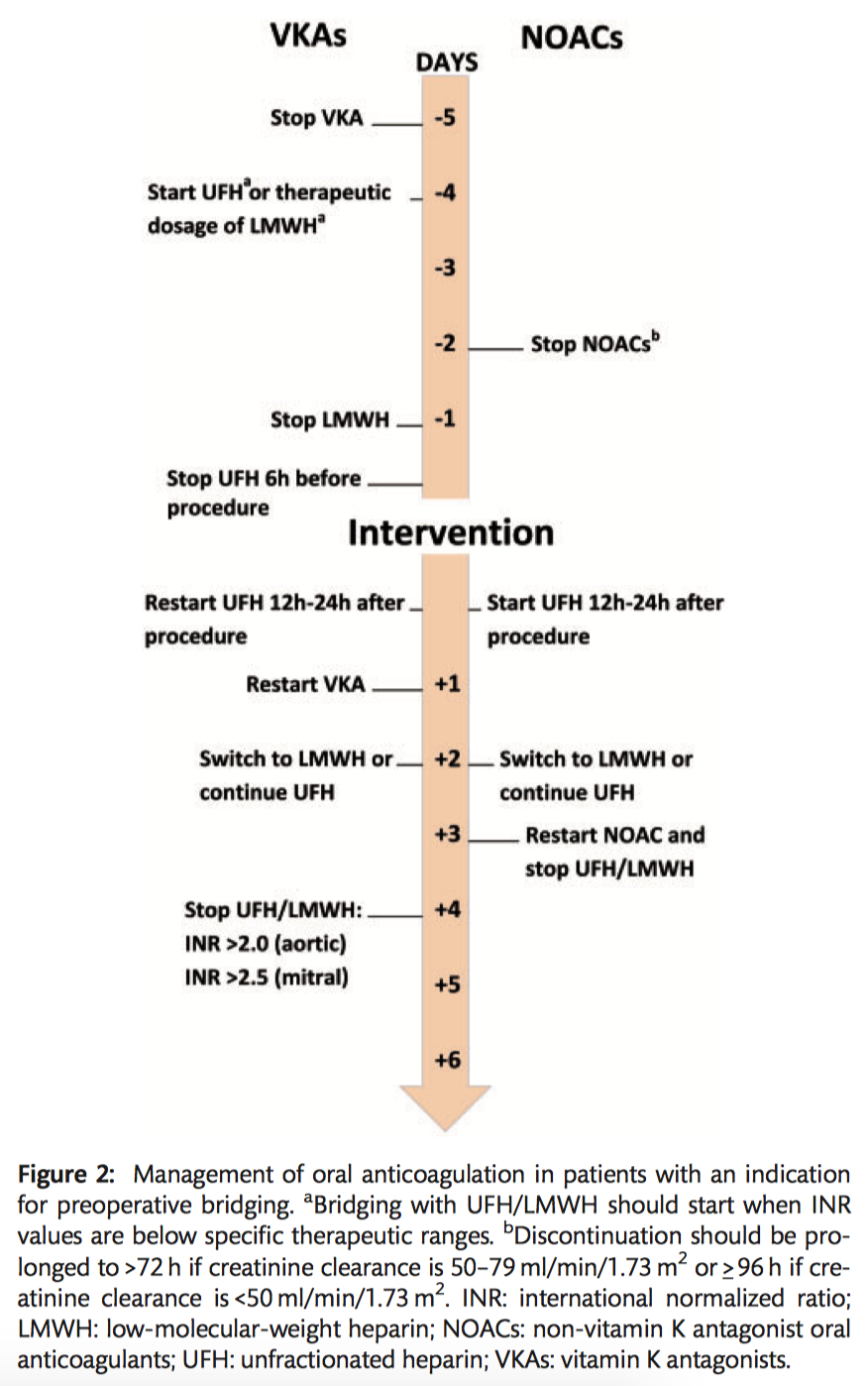
- It is recommended that VKAs be discontinued 5 days prior to surgery to aim for an INR<1.5 on the day of elective cardiac surgery (Class IC).
- For patients on NOACs, preoperative discontinuation of therapy is recommended at least 48-96 hours prior to surgery, depending on renal function and the agent (Class IC).
- Bridging of OAC is recommended in patients with any of the following indications (Class IC):
- Mechanical prosthetic heart valve
- AF with moderate-severe mitral stenosis
- AF with a CHA2DS2-VASc score >4
- Acute thrombotic event within preceding 4 weeks
- Bridging of OACs should be considered in patients with a high thromboembolic risk (Class IIa C).
- Bridging with UFH is recommended (Class IB).
- Bridging with subcutaneous LMWH should be considered as an alternative to bridging with UFH (Class IIa B).
- Bridging with fondaparinux is not recommended (Class III C).
- In patients who preoperatively bridged with UFH, it is recommended that UFH be stopped 6 hours before surgery (Class I C).
- In patients who are preoperatively bridged with therapeutic LMWH, it is recommended that they be given the last dose 24 hours before surgery (Class I B).
Postoperative management of oral anticoagulants and indications for long-term antithrombotic treatments
Postoperative bridging and (re)starting oral anticoagulation
- In patients with an indication for postoperative therapeutic bridging, it is recommended to start UFH 12–24 hours after surgery (Class I C).
- LMWH should be considered as an alternative bridging strategy to UFH 24-48 hours after surgery (Class IIa C).
- It is recommended to reinitiate VKAs on the first postoperative day (Class IC).
- Delaying the restarting of NOACs for 72 hours after surgery should be considered (Class IIa C).
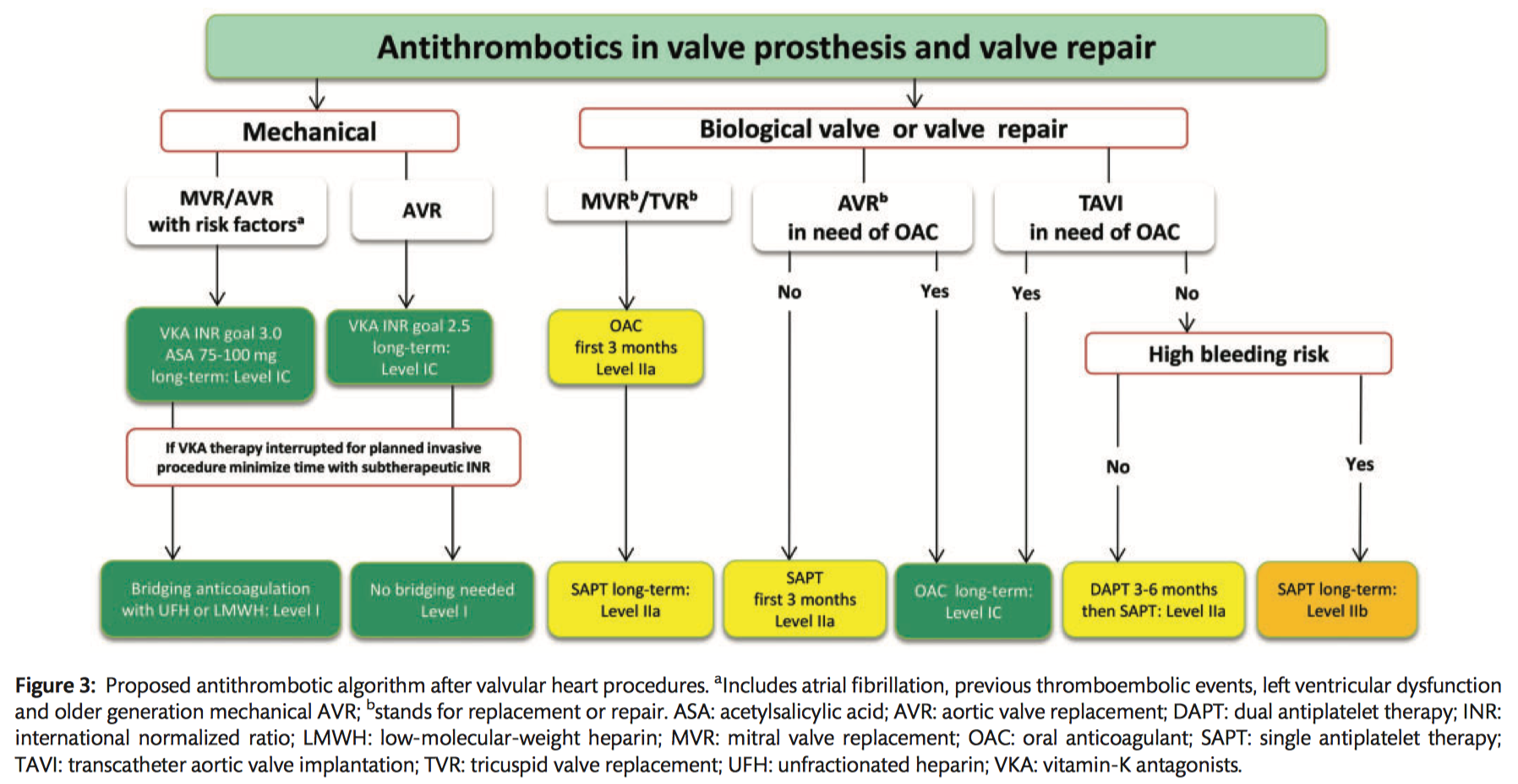
Mechanical prostheses
- Lifelong anticoagulation using a VKA is recommended for all patients (Class I B).
- NOACs are not recommended in patients with a mechanical valve prosthesis (Class III B).
- The addition of low dose ASA (75-100mg/day) to VKA should be considered in the case of concomitant atherosclerotic disease (Class IIa C).
- The addition of lifelong low dose ASA (75–100 mg/day) to VKA should be considered after thromboembolism despite an adequate INR (Class IIa C).
- Triple therapy comprising VKAs, ASA (75–100 mg/day) and clopidogrel (75 mg/day) should be considered for a duration of 1 month after ACS or recent stent implantation, followed by VKAs and low ASA (75–100 mg/day) or clopidogrel (75 mg/day) (Class IIa C).
- INR self management is recommended provided that appropriate training and quality control are performed (Class I B).
Bioprostheses
- Oral anticoagulation is recommended on a lifelong basis for patients with surgically or transcatheter implanted bioprostheses who have other indications for anticoagulation (Class I C).
- Oral anticoagulation may be considered for the first 3 months after surgical implantation of an aortic bioprosthesis (Class IIb B).
- Low-dose ASA (75–100 mg/day) should be considered for the first 3 months after surgical implantation of an aortic bioprosthesis or valve-sparing aortic surgery (Class IIa B).
- Oral anticoagulation using a VKA should be considered for the first 3 months after mitral or tricuspid valve repair or bioprosthetic valve replacement (Class IIa C).
TAVI
- DAPT should be considered for the first 3–6 months after TAVI, followed by lifelong ASA in patients who do not need OACs for other reasons (Class IIa C).
- ASA monotherapy may be considered after TAVI in cases where there is a high risk for bleeding (Class IIb C).
Other indications
- In patients undergoing cardiac surgery with a preoperative indication for oral anticoagulation, it is recommended that the preoperative regimen of VKAs or NOACs be restarted after surgery (Class I C).
Recommendations for prevention in and treatment of patients with atrial fibrillation
Preoperatively:
- Perioperative low-dose oral beta-blocker therapy, starting 2–3 days before cardiac surgery, should be considered for the prevention of POAF (Class IIa B).
- If beta-blockers are initiated preoperatively, careful up-titration, according to blood pressure and heart rate, starting several days before surgery, is recommended (Class I C).
- Perioperative amiodarone, starting 5–6 days before cardiac surgery, may be considered for the prevention of POAF (Class IIb A).
Postoperatively:
- In patients with postoperative haemodynamically stable POAF, rhythm control is recommended (Class I B).
- In patients with postoperative haemodynamically stable and asymptomatic POAF, rate control should be considered (Class IIa B).
- In patients with postoperative haemodynamically unstable POAF, cardioversion and antiarrhythmic drugs to restore sinus rhythm are recommended (Class I B).
- Anticoagulation with therapeutic doses of UFH or LMWH should be considered within 12–48 hours of AF in patients with POAF, balancing the risks for stroke and surgical bleeding (Class IIa C).
- In patients with POAF at discharge, it is recommended to initiate OAC therapy and continue for at least 4 weeks (or longer), depending on the CHA2DS2-VASc risk score (Class I B).
Management of patients with renin-angiotensin-aldosterone system inhibitors and indications for long-term treatment
Preoperatively:
- It is recommended to discontinue ACEIs and ARBs preoperatively in patients undergoing cardiac surgery (Class I C).
- In patients with preoperative uncontrolled arterial hypertension, switching long-acting ACEI or ARB treatment to short-acting ACEIs should be considered (Class IIa C).
Postoperatively:
- It should be considered to start short-acting ACEIs at a low dose no earlier than 48 hours after cardiac surgery in patients with a reduced LVEF (<40%) and an eGFR >30 ml/min/1.73m2 (Class IIa C).
- In ACEI-intolerant patients, an ARB is recommended in patients with a reduced LVEF (<40%) and an eGFR >30 ml/min/1.73 m2 (Class I A).
- Long-term optimal-dose ACEI or ARB treatment is recommended after cardiac surgery in patients with reduced LVEF (<40%) and an eGFR >30 ml/min/1.73 m2 (Class I A).
- Sacubitril/valsartan is recommended as a replacement for an ACEI in ambulatory patients with reduced LVEF (<40%) who remain symptomatic despite optimal treatment with an ACEI, a beta-blocker and aldosterone antagonists (Class I B).
- Long-term aldosterone antagonist addition to beta-blockers and ACEI therapy is recommended after cardiac surgery in patients with HF and a reduced LVEF (<35%), an eGFR >30 ml/min/1.73 m2 and without hyperkalaemia (>5.0 mEG/l) (Class I A).
Management of treatment with beta-blockers in perioperative settings
Preoperatively:
- It should be considered to continue beta-blocker therapy prior to cardiac surgery (Class IIa B).
Postoperatively:
- Postoperative long term beta-blocker therapy is recommended in patients with a recent MI or reduced LVEF (<35%) (Class I A).
Management of patients with dyslipidaemia
Preoperative period
- It is not recommended to initiate statin therapy shortly before cardiac surgery (Class III A).
- Continuing statin therapy before cardiac surgery should be considered (Class IIa C).
Postoperative period
- LDL-C is recommended as the primary target (Class I A).
- Intense or maximally tolerated statin therapy is recommended in patients after CABG surgery to reach the LDL-C target <70 mg/dL (1.8 mmol/l) or >50% LDL-C reduction if the baseline LDL-C is between 1.8–3.5 mmol/l (70–135 mg/dl) (Class I A).
- In patients after CABG surgery in whom the LDL-C target <70 mg/dL (1.8 mmol/l) is not reached by using statin therapy, a combination of a statin with a cholesterol absorption inhibitor (ezetimibe) should be considered (Class IIa B).
- In patients after CABG surgery who have a persistently high LDLC (>140 mg/dl or 3.6 mmol/l) level, despite treatment with the maximal tolerated statin dose (in combination with ezetimibe), a PCSK9 inhibitor should be considered (Class IIa B).
Recommendations for stress ulcer prophylaxis
- The prophylactic use of a PPI for patients undergoing cardiac surgery should be considered to reduce gastric complications (Class IIa B).
- The prophylactic use of an H2 antagonist for patients undergoing cardiac surgery may be considered to reduce gastric complications (Class IIb B).
Recommendation for the routine use of steroids
- The routine use of prophylactic steroids for patients undergoing cardiac surgery is not recommended (Class IIIA).
Recommendations for antibiotic prophylaxis
- In elective patients undergoing cardiac surgery who are S. aureus carriers, mupirocin twice daily intranasally is recommended, starting 4 days before surgery (Class I B).
- In elective patients undergoing cardiac surgery with an unknown intranasal S. aureus colonization status, a strategy of testing well in advance of cardiac surgery to allow the appropriate preoperative duration of mupirocin eradication treatment in colonized patients should be considered over routine mupirocin treatment (Class IIa C).
- Primary SAP is recommended to prevent infectious complications (Class I A).
Timing
- Administration of the first dose of antimicrobial therapy within the 60 min before surgical incision is recommended (Class I B).
- Administration of vancomycin and fluoroquinolones within the 120 min before surgical incision is recommended (Class I B).
Dosing
- It is recommended to use SAP according to standardized doses (Class I B).
Duration
- It should be considered that the optimal duration of SAP is 24 hours and should not exceed 48 hours following cardiac surgery (Class IIa A).
- Intraoperative redosing either with half a dose or a full dose depending on the antibiotic that is used, the length of operation, BMI and renal function should be considered to obtain adequate serum and tissue concentrations of the antimicrobial agent if the duration of the procedure exceeds two half-lives of the antimicrobial treatment (Class IIa B).
- Intraoperative redosing either with half a dose or a full dose depending on the antibiotic that
is used, the length of the operation, BMI and renal function should be considered to obtain
adequate serum and tissue concentrations of the antimicrobial agent if there is haemodilution during CPB or excessive blood loss (Class IIa B).
Choice
- First-line treatment with cefazolin or cefuroxime is recommended (Class I A).
- Clindamycin or vancomycin are recommended in patients with a documented ß-lactam allergy (Class I B).
- Vancomycin should be considered for prophylaxis in patients known to be colonized with MRSA (Class IIa B).
Treatment options in managing perioperative pain
- Multimodal analgesia is recommended over single-technique analgesia (Class I B).
- It is recommended that adult patients undergoing cardiac surgery undergo routine assessment of pain presence and severity for optimal analgesia (Class I B).
- It is recommended that an anaesthesia plan including a halogenated agent (isoflurane, desflurane or sevoflurane) is used in CABG patients (Class I B).
- The use of epidural analgesia may be considered after careful consideration of benefits and risks (Class IIb B).
- Preoperative intrathecal morphine administration may be considered to reduce postoperative opioid consumption (Class IIb B).
- The paravertebral block may be considered as an alternative to neuraxial techniques (Class IIb B).
- Parasternal continuous infusion of analgesia is not recommended in cardiac surgery (Class III B).
- Perioperative remifentanil infusion should be considered in all patients undergoing cardiac surgery (Class IIa B).
- PAC should be considered over a nurse-driven protocol (Class IIa B).
- IV opioids plus IV paracetamol should be considered as firstline treatment for postoperative pain in the ICU after cardiac surgery (Class IIa B).
- Routine NSAIDs are not recommended as first-line agents in unselected cardiac surgical patients (Class III A).
- Short-term low-dose NSAIDs may be considered as second-line agents in selected patients with a low risk of postoperative AKI and no contraindications to NSAIDs (Class IIb B).
- COX-2 inhibitors are not recommended in cardiac surgical patients (Class III A).
- It may be considered to start gabapentin or pregabalin before surgery as postoperative analgesic adjuvants (Class IIb B).
- Ketamine may be considered a postoperative analgesic adjuvant (Class IIb B).
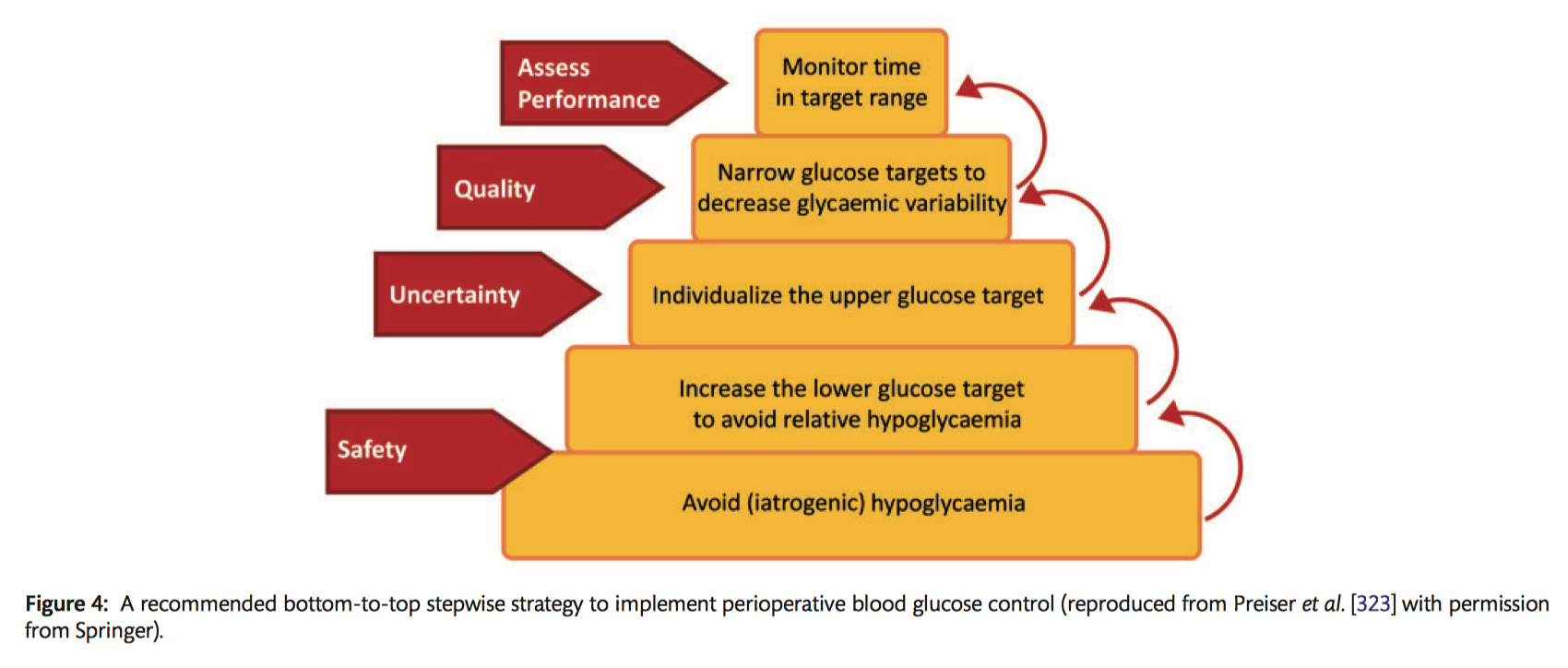
Specific recommendations for perioperative blood glucose management
Preoperatively:
- It is recommended that oral antidiabetics and long-acting subcutaneous insulin be omitted the day before surgery (Class I C).
- It should be considered that preoperative short-acting subcutaneous insulin is used while patients await surgery to maintain blood glucose levels between 120–180 mg/dl (6.7–10 mmol/l), with a check every 4 hours (Class IIa C).
Intraoperatively:
- It should be considered that non-DM patients have a small (5 IU) bolus IV of insulin if the blood glucose level is >180 mg/dl (>10 mmol/l), as well as hourly checks (Class IIa C).
- It should be considered that in non-DM patients a continuous IV insulin infusion is started to maintain a blood glucose of 150–180 mg/dl (8.3–10 mmol/l) during surgery if blood glucose is persistently >180 mg/dl (<10 mmol/l) (Class IIa B).
- In diabetic patients, it is recommended that a continuous IV insulin infusion is started at the beginning of surgery and continued throughout to maintain a blood glucose level >150 (>8.3 mmol/l) and <180 mg/dl (<10 mmol/l) (Class I B).
ICU:
- With diabetic and non-DM patients, continuous IV insulin infusion is recommended if the blood glucose level is >180 mg/dl (>10 mmol/l) for a target of 150–180 mg/dl (8.3–10 mmol/l) during the ICU stay (Class I B).
- It is recommended that blood glucose levels are checked hourly if not stable and every 4 hours if stable during the ICU stay (Class I C).
After ICU:
- It should be considered to start a combination of short-acting and long-acting subcutaneous insulin at 50% of the total previous 24-hour insulin dose (in ICU) and then titrated (Class IIa C).
- Checking the blood glucose level every 4 hours and adjusting insulin doses to a target of 150–180 mg/dl (8.3–10 mmol/l) should be considered (Class IIa C).
- It may be considered to restart oral antidiabetics at 50% of the preoperative dose when the patient is on oral feeding (Class IIb C).
At discharge:
- It is recommended that patients with DM or specifically, de novo DM, consult a diabetic specialist before discharge (Class I C).